Carbohydrate Research
2006-07-24
On the nitrile effect in L-rhamnopyranosylation.
David Crich, Mitesh Patel
Index: Carbohydr. Res. 341(10) , 1467-75, (2006)
Full Text: HTML
Abstract
It is shown that the use of 5% acetonitrile or propionitrile in dichloromethane functions to increase the beta-selectivity of a number of L-rhamnopyranosylation reactions conducted by the thioglycoside method with activation by the 1-benzenesulfinyl piperidine/trifluoromethanesulfonic anhydride couple. The use of more significant quantities of acetonitrile or propionitrile results in the formation of complex reaction mixtures containing little coupled product, but from which Ritter-type products can be isolated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
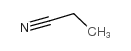 |
propionitrile
CAS:107-12-0 |
C3H5N |
Related Articles:
More...
|
Functionalization of graphene with self-doped conducting pol...
2015-10-01 [J. Colloid. Interface Sci. 455 , 63-70, (2015)] |
|
G protein-coupled estrogen receptor (GPER) mediates NSCLC pr...
2015-04-01 [Med. Oncol. 32(4) , 104, (2015)] |
|
In vitro metabolic conversion of the organic breakdown produ...
2015-08-30 [J. Sci. Food Agric. 95 , 2244-51, (2015)] |
|
High IL-17E and low IL-17C dermal expression identifies a fi...
2014-01-01 [PLoS ONE 9(8) , e105008, (2014)] |
|
Critical period for dopaminergic neuroprotection by hormonal...
2015-02-01 [Neurobiol. Aging 36(2) , 1194-208, (2015)] |