High-temperature hydrodechlorination of ozone-depleting chlorodifluoromethane (HCFC-22) on supported Pd and Ni catalysts.
Jeong-Myeong Ha, Daewoo Kim, Jaehoon Kim, Byoung Sung Ahn, Yunje Kim, Jeong Won Kang
Index: J. Environ. Sci. Health. A. Tox. Hazard. Subst. Environ. Eng. 46(9) , 989-96, (2011)
Full Text: HTML
Abstract
The hydrodechlorination of chlorodifluoromethane (HCFC-22) was performed by a catalytic reaction and noncatalytic thermal decomposition at high temperatures of 400-800 °C. After 47 h of time-on-stream on a supported palladium (Pd) catalyst, the gas phase composition of difluoromethane (HFC-32) is 41.0%, with 4.9% of the HCFC-22 remaining, indicating the conversion of up to 95.1% of HCFC-22. The supported nickel catalyst's deactivation is significant as it exhibits the low conversion of HCFC-22 under the same reaction conditions. The deactivation of the catalyst is caused by the polymerization of adsorbed methyl radicals, which competes with the formation of HFC-32. With concentrated reactants at high reaction temperatures, there was an increase in the catalytic activity; however, unwanted tar, methane, and trifluoromethane (HFC-23) by-products are also produced. The use of catalyst suppresses the formation of these by-products. Considering the compositions of the products of the catalytic and noncatalytic reactions, we demonstrate that the use of the supported-metal catalysts and hydrogen flow suppresses tar formation and lowers the required reaction temperature.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
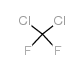 |
Dichlorodifluoromethane
CAS:75-71-8 |
CCl2F2 |
|
Simultaneous determination of CFC-11, CFC-12 and CFC-113 in ...
2009-01-01 [Talanta 80(2) , 959-66, (2009)] |
|
Background monitoring and long-range transport of atmospheri...
2001-07-01 [Environ. Monit. Assess. 70(1-2) , 47-56, (2001)] |
|
Standards development of global warming gas species: methane...
2004-05-01 [Environ. Sci. Technol. 38(9) , 2685-92, (2004)] |
|
In vitro approaches to evaluate placental drug transport by ...
2011-02-01 [Basic Clin Pharmacol Toxicol. 108(2) , 138-45, (2011)] |
|
Monochromatic soft-x-ray-induced reactions of CF2Cl2 adsorbe...
2011-11-02 [J. Phys. Condens. Matter 23(43) , 435011, (2011)] |