Radiolabeling of multimeric neurotensin(8-13) analogs with the short-lived positron emitter fluorine-18.
Christina Hultsch, Mathias Berndt, Ralf Bergmann, Frank Wuest
Index: Appl. Radiat. Isot. 65(7) , 818-26, (2007)
Full Text: HTML
Abstract
Three methods for (18)F-labeling of dimeric and tetrameric neurotensin(8-13) derivatives were evaluated with respect to the labeling yield and the required peptide amounts. Labeling using N-succinimidyl-4-[(18)F]fluorobenzoate ([(18)F]SFB) gave low radiochemical yield for the dimeric peptides. Coupling of the tetramer with [(18)F]SFB was not successful. High yields were obtained for labeling of the aminooxy-functionalized neurotensin(8-13) dimer using 4-[(18)F]fluorobenzaldehyde ([(18)F]FBA) whilst coupling of the corresponding tetramer gave only low yields. Labeling of sulfydryl-functionalized neurotensin(8-13) derivatives using the maleinimide 4-[(18)F]fluorobenzaldehyde-O-[6-(2,5-dioxo-2,5-dihydro-pyrrol-1-yl)-hexyl]-oxime ([(18)F]FBAM) resulted in high radiochemical yields for both, the dimer and the tetramer. Therefore, [(18)F]FBAM seems to be the most suitable (18)F-labeling agent for multivalent neurotensin(8-13) derivatives.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
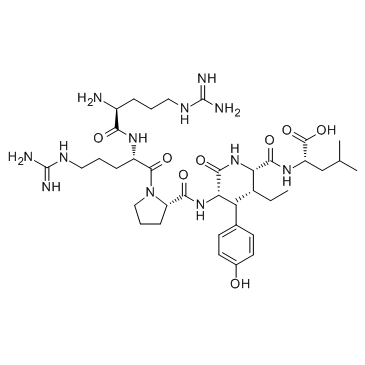 |
Neurotensin (8-13) acetate salt
CAS:60482-95-3 |
C38H64N12O8 |
|
In vivo behavioral effects of stable, receptor-selective neu...
2005-03-01 [Neuropharmacology 48(3) , 417-25, (2005)] |
|
The neurotensin fragment AcNT(8-13) inhibits lowering of int...
2002-09-01 [Am. J. Physiol. Heart Circ. Physiol. 283(3) , H933-40, (2002)] |
|
Gq/11-induced and spontaneous waves of coordinated network a...
2005-02-16 [J. Neurosci. 25(7) , 1737-49, (2005)] |
|
Radionuclide imaging of small-cell lung cancer (SCLC) using ...
2006-05-01 [Nucl. Med. Biol. 33(4) , 505-12, (2006)] |
|
Separation and determination of peptide hormones by capillar...
2008-09-15 [Anal. Biochem. 380(2) , 297-302, (2008)] |