Hofmeister phenomena in nonaqueous media: the solubility of electrolytes in ethylene carbonate.
Niccolò Peruzzi, Barry W Ninham, Pierandrea Lo Nostro, Piero Baglioni
Index: J. Phys. Chem. B 116(49) , 14398-405, (2012)
Full Text: HTML
Abstract
The solubility of some potassium salts (KF, KCl, KBr, KI, KNO(3), KClO(4), KSCN, and KSeCN) in ethylene carbonate (EC) was determined at different temperatures with an inductively coupled plasma atomic emission spectrometer. From the solubility measurements, the thermodynamic parameters ΔG, ΔH, and ΔS, of solution and of solvation, were calculated. Measurements were carried out via XRD, ATR, and FTIR to determine the effect of each salt on the properties of the solvent. The open question of whether specific ion (Hofmeister) effects are restricted to hydration peculiar to water is resolved. As for water, the effects are due to solute (ion, dipolar) induced solvent structure not accounted for by electrostatic forces. Cooperative quantum mechanical forces are necessary to understand the phenomena.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
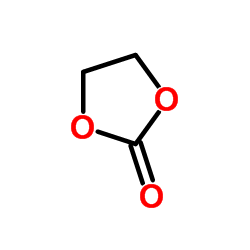 |
Ethylene carbonate
CAS:96-49-1 |
C3H4O3 |
|
Screening and quantification of pesticide residues in fruits...
2014-11-01 [Anal. Bioanal. Chem , (2014)] |
|
Design and synthesis of a novel pre-column derivatization re...
2015-12-10 [J. Pharm. Biomed. Anal. 116 , 71-9, (2015)] |
|
Interaction of High Flash Point Electrolytes and PE-Based Se...
2015-01-01 [Int. J. Mol. Sci. 16 , 20258-76, (2015)] |
|
Electron paramagnetic resonance imaging for real-time monito...
2015-01-01 [Nat. Commun. 6 , 6276, (2015)] |
|
Effects of Solute-Solvent Hydrogen Bonding on Nonaqueous Ele...
2015-08-06 [J. Phys. Chem. Lett. 6 , 2888-91, (2015)] |