Total synthesis of (+)-brasilenyne. Application of an intramolecular silicon-assisted cross-coupling reaction.
Scott E Denmark, Shyh-Ming Yang
Index: J. Am. Chem. Soc. 126(39) , 12432-40, (2004)
Full Text: HTML
Abstract
The first enantioselective total synthesis of (+)-brasilenyne (1) has been achieved in 19 linear steps, with 5.1% overall yield from l-(S)-malic acid. The construction of the oxonin core containing a 1,3-cis,cis diene unit was accomplished with a tandem ring-closing metathesis/silicon-assisted intramolecular cross-coupling reaction. In addition, a key propargylic stereogenic center was created through a novel, highly diastereoselective ring opening of a 1,3-dioxolanone promoted by TiCl(4). This reaction proceeded through an oxocarbenium ion intermediate and the asymmetric induction was fully controlled by l-malic acid residue. The C(8) stereogenic center was set by a reagent-controlled asymmetric allylboration.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
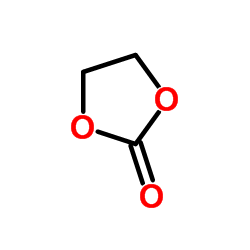 |
Ethylene carbonate
CAS:96-49-1 |
C3H4O3 |
|
Screening and quantification of pesticide residues in fruits...
2014-11-01 [Anal. Bioanal. Chem , (2014)] |
|
Design and synthesis of a novel pre-column derivatization re...
2015-12-10 [J. Pharm. Biomed. Anal. 116 , 71-9, (2015)] |
|
Interaction of High Flash Point Electrolytes and PE-Based Se...
2015-01-01 [Int. J. Mol. Sci. 16 , 20258-76, (2015)] |
|
Electron paramagnetic resonance imaging for real-time monito...
2015-01-01 [Nat. Commun. 6 , 6276, (2015)] |
|
Effects of Solute-Solvent Hydrogen Bonding on Nonaqueous Ele...
2015-08-06 [J. Phys. Chem. Lett. 6 , 2888-91, (2015)] |