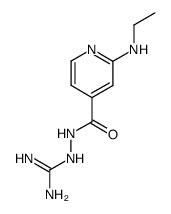
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

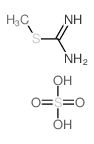
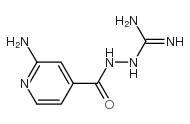

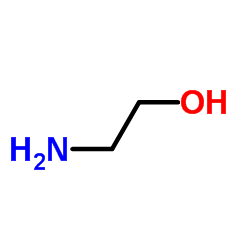
![2-[[4-(3-amino-1H-1,2,4-triazol-5-yl)pyridin-2-yl]amino]ethanol Structure](https://image.chemsrc.com/caspic/448/77314-62-6.png)