Mitochondria maintain maturation and secretion of lipoprotein lipase in the endoplasmic reticulum.
Karin Osibow, Sasa Frank, Roland Malli, Rudolf Zechner, Wolfgang F Graier
Index: Biochem. J. 396(1) , 173-82, (2006)
Full Text: HTML
Abstract
Considering the physiological Ca2+ dynamics within the ER (endoplasmic reticulum), it remains unclear how efficient protein folding is maintained in living cells. Thus, utilizing the strictly folding-dependent activity and secretion of LPL (lipoprotein lipase), we evaluated the impact of ER Ca2+ content and mitochondrial contribution to Ca2+-dependent protein folding. Exhaustive ER Ca2+ depletion by inhibition of sarcoplasmic/endoplasmic reticulum Ca2+-ATPases caused strong, but reversible, reduction of cell-associated and released activity of constitutive and adenovirus-encoded human LPL in CHO-K1 (Chinese-hamster ovary K1) and endothelial cells respectively, which was not due to decline of mRNA or intracellular protein levels. In contrast, stimulation with the IP3 (inositol 1,4,5-trisphosphate)-generating agonist histamine only moderately and transiently affected LPL maturation in endothelial cells that paralleled a basically preserved ER Ca2+ content. However, in the absence of extracellular Ca2+ or upon prevention of transmitochondrial Ca2+ flux, LPL maturation discontinued upon histamine stimulation. Collectively, these data indicate that Ca2+-dependent protein folding in the ER is predominantly controlled by intraluminal Ca2+ and is largely maintained during physiological cell stimulation owing to efficient ER Ca2+ refilling. Since Ca2+ entry and mitochondrial Ca2+ homoeostasis are crucial for continuous Ca2+-dependent protein maturation in the ER, their pathological alterations may result in dysfunctional protein folding.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
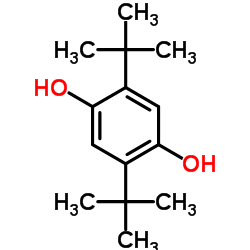 |
2,5-Di-tert-butylhydroquinone
CAS:88-58-4 |
C14H22O2 |
|
Discovery of novel SERCA inhibitors by virtual screening of ...
2011-05-01 [Eur. J. Med. Chem. 46 , 1512-23, (2011)] |
|
Effect of methoxychlor on Ca(2+) movement and viability in M...
2015-03-01 [Basic Clin Pharmacol Toxicol. 111(4) , 224-31, (2012)] |
|
Two distinct calcium pools in the endoplasmic reticulum of H...
2011-04-01 [Biochem. J. 435(1) , 227-35, (2011)] |
|
Effects of high-affinity inhibitors on partial reactions, ch...
2008-04-01 [Mol. Pharmacol. 73(4) , 1134-40, (2008)] |
|
Functional relevance of the de novo coupling between hTRPC1 ...
2008-04-01 [Cell. Signal. 20(4) , 737-47, (2008)] |