| Structure | Name/CAS No. | Articles |
|---|---|---|
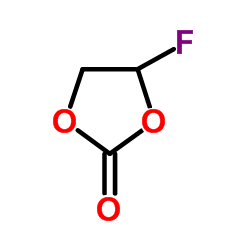 |
4-Fluoro-1,3-dioxolan-2-one
CAS:114435-02-8 |
|
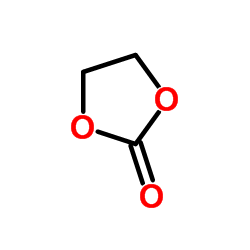 |
Ethylene carbonate
CAS:96-49-1 |
|
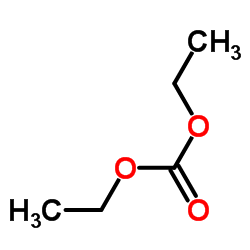 |
Ethyl carbonate
CAS:105-58-8 |
|
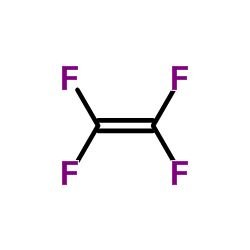 |
poly(tetrafluoroethylene)
CAS:9002-84-0 |