| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(S)-(?)-Perillyl alcohol
CAS:18457-55-1 |
|
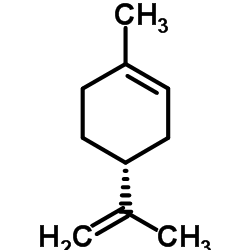 |
(+)-Limonene
CAS:5989-27-5 |
|
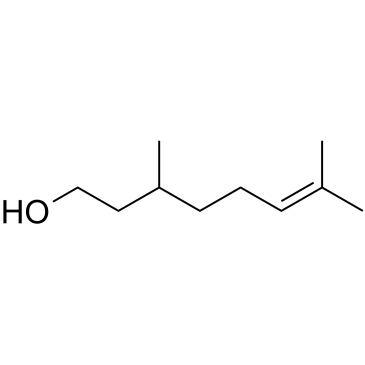 |
Citronellol
CAS:106-22-9 |
|
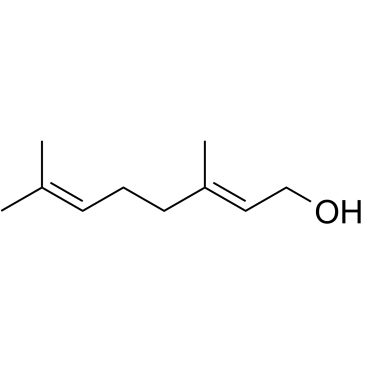 |
Geraniol
CAS:106-24-1 |
|
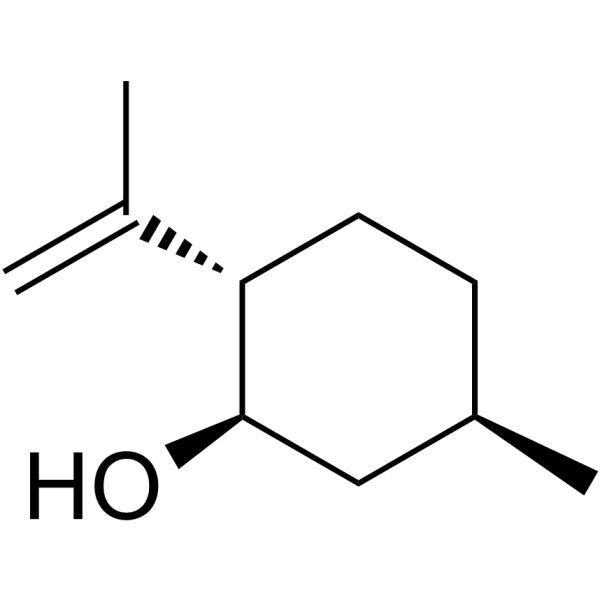 |
ISOPULEGOL
CAS:89-79-2 |
|
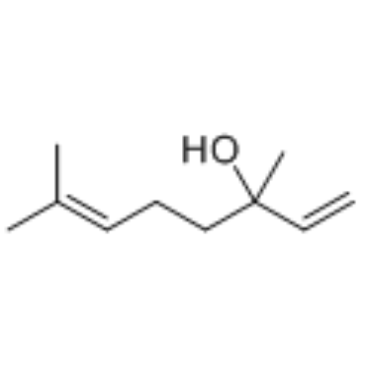 |
Linalool
CAS:78-70-6 |
|
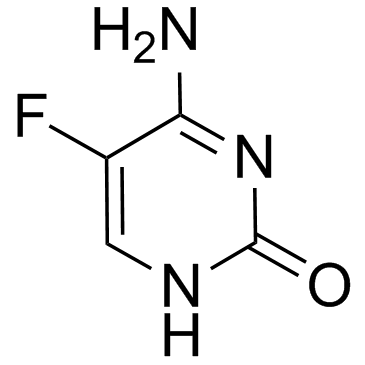 |
5-Flucytosine
CAS:2022-85-7 |