| Structure | Name/CAS No. | Articles |
|---|---|---|
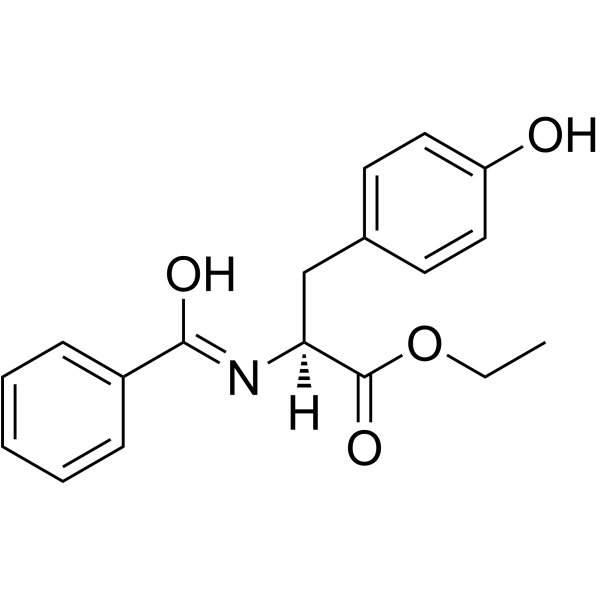 |
Bz-Tyr-OEt; BTEE
CAS:3483-82-7 |
|
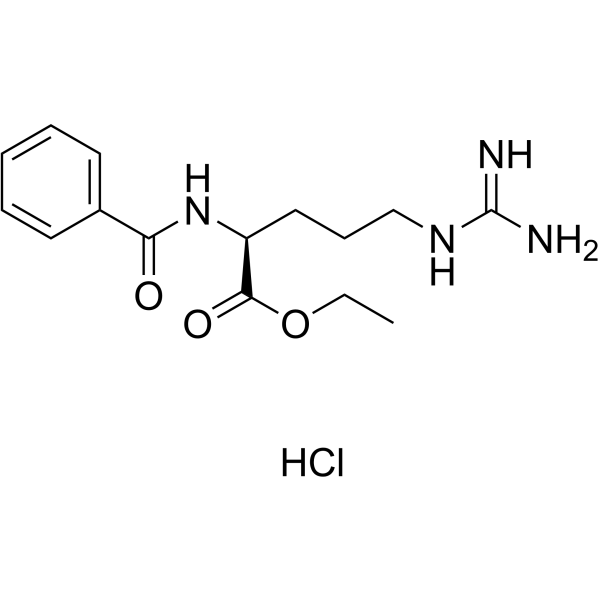 |
Bz-Arg-OEt��HCl
CAS:2645-08-1 |