Studies on chymotrypsin-like catalysis by synthetic peptides.
M J Corey, E Hallakova, K Pugh, J M Stewart
Index: Appl. Biochem. Biotechnol. 47(2-3) , 199-210; discussion 210-2, (1994)
Full Text: HTML
Abstract
The synthetic peptide Chymohelizyme-1 (CHZ-1) exhibits esterase activity against carbobenzoxytyrosine p-nitrophenyl ester (ZTONP), carbobenzoxyalanine p-nitrophenyl ester (ZAONP), and t-butyloxy-carbonyltyrosine p-nitrophenyl ester (BocTONP). However, earlier reports of catalytic activity against less labile esters and amides have proven to be incorrect. The major reason for the errors appears to have been the omission of certain controls in the previous work. Although the catalytic triad does not appear to be functioning as designed, the catalytic activity of CHZ-1 does depend on the integrity of its primary structure. The pH dependence of hydrolysis of ZTONP points to general-base catalysis, whereas a preference for hydrophobic substrates suggest that the structure of CHZ-1 is performing some other role in assisting catalysis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
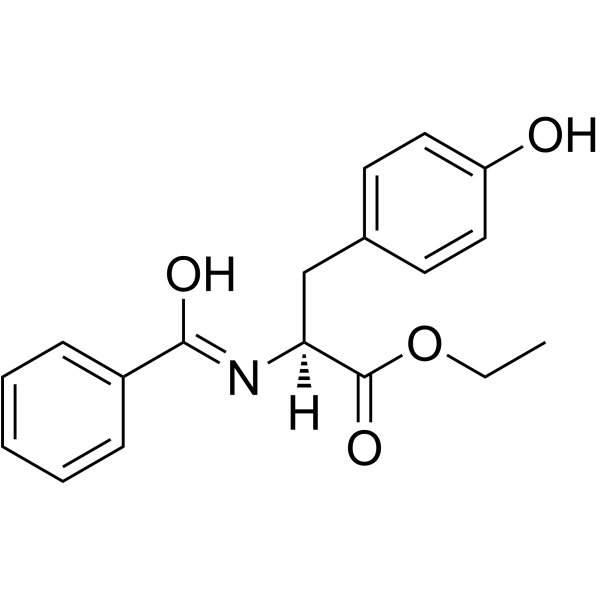 |
Bz-Tyr-OEt; BTEE
CAS:3483-82-7 |
C18H19NO4 |
|
Polymer enzyme conjugates as chiral ligands for sharpless di...
2015-01-02 [ChemBioChem. 16(1) , 83-90, (2014)] |
|
The protective role of the Bowman-Birk protease inhibitor in...
2015-08-01 [Food Funct. 6 , 2626-35, (2015)] |
|
Synthesis of a monolithic, micro-immobilised enzyme reactor ...
2012-07-01 [Anal. Bioanal. Chem 403(9) , 2655-63, (2012)] |
|
Kinetic analysis of deactivation of immobilized alpha-chymot...
1999-10-20 [Biotechnol. Bioeng. 65(2) , 170-5, (1999)] |
|
NF-kappa B modulates TNF-alpha production by alveolar macrop...
2000-02-01 [J. Immunol. 164(3) , 1588-94, (2000)] |