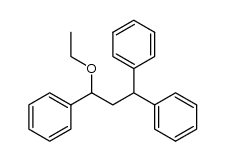
|
~15% |
|
~6% |
|
~% |
|
~16%
Detail
|
|
~% |
|
~10% |
|
~5% |
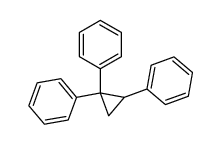
![[methoxy(phenyl)methyl]benzene Structure](https://image.chemsrc.com/caspic/392/1016-09-7.png)
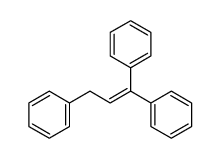



![Benzene,1,1'-[(1,1-dimethylethoxy)methylene]bis Structure](https://image.chemsrc.com/caspic/249/28567-35-3.png)
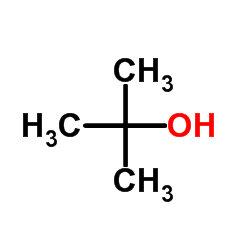

![[ethoxy(phenyl)methyl]benzene Structure](https://image.chemsrc.com/caspic/207/5670-78-0.png)