Design and synthesis of marine fungal phthalide derivatives as PPAR-γ agonists.
Bin Xiao, Jun Yin, Minhi Park, Juan Liu, Jian Lin Li, Eun La Kim, Jongki Hong, Hae Young Chung, Jee H Jung
Index: Bioorg. Med. Chem. 20(16) , 4954-61, (2012)
Full Text: HTML
Abstract
On the basis of a marine fungal phthalide (paecilocin A) skeleton, we synthesized 20 analogs and evaluated them for peroxisome proliferator-activated receptor gamma (PPAR-γ) binding and activation. Among these analogs, 6 and 7 had significant PPAR-γ binding activity, and 7 showed further PPAR-γ activation in rat liver Ac2F cells. In docking simulation, 7 formed H bonds with key amino acid residues of the PPAR-γ binding domain, and the overall positioning was similar to rosiglitazone. This new phthalide derivative is considered an interesting new molecular class of PPAR-γ ligands.Copyright © 2012 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
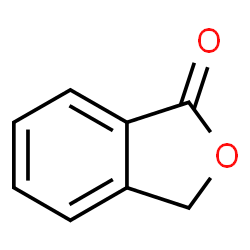 |
Phthalide
CAS:87-41-2 |
C8H6O2 |
|
Development of an Immunosensor for Determination of the Fung...
2015-07-22 [J. Agric. Food Chem. 63 , 6325-30, (2015)] |
|
Palladium-catalyzed cascade aryl addition/intramolecular lac...
2010-09-03 [J. Org. Chem. 75(17) , 6043-5, (2010)] |
|
Phthalide derivatives with antifungal activities against the...
2011-11-01 [J. Antibiot. 64(11) , 723-7, (2011)] |
|
Metabolic profiling of GuanXin II prescription based on meta...
2011-03-25 [J. Pharm. Biomed. Anal. 54(4) , 789-98, (2011)] |
|
Adulticidal activity of phthalides identified in Cnidium off...
2011-08-10 [J. Agric. Food Chem. 59(15) , 8193-8, (2011)] |