Organic Letters
2011-07-01
β-Olefination of 2-alkynoates leading to trisubstituted 1,3-dienes.
Mathias J Jacobsen, Erik Daa Funder, Jacob R Cramer, Kurt V Gothelf
Index: Org. Lett. 13(13) , 3418-21, (2011)
Full Text: HTML
Abstract
A phosphine-mediated olefination of 2-alkynoates with aldehydes forming 1,3-dienes with high E-selectivity and up to 88% yield is described. Reaction conditions are optimized and reactions are demonstrated for various aryl, alkyl, and alkenyl aldehydes and for ethyl 2-alkynoates with different substituents in the δ-position. Proof of concept is shown for the generation of a β,γ-unsaturated lactone by intramolecular olefination, and furthermore the use of the generated 1,3-dienes in the Diels-Alder reaction has been demonstrated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
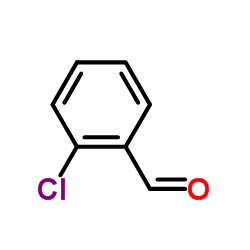 |
2-Chlorobenzaldehyde
CAS:89-98-5 |
C7H5ClO |
Related Articles:
More...
|
Acetalization allows the photoheterolysis of the Ar-Cl bond ...
2012-10-19 [J. Org. Chem. 77(20) , 9094-101, (2012)] |
|
Experimental and theoretical studies of the products of addi...
2015-07-01 [Acta Crystallogr. C Struct. Chem. 71 , 554-63, (2015)] |
|
Vibrational spectra of partially oriented molecules having t...
2005-05-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 61(7) , 1661-70, (2005)] |
|
Analysis of the aneuploidy inducing capacity of 2-chlorobenz...
1991-07-01 [Mutagenesis 6(4) , 303-5, (1991)] |
|
Synthesis of ionones and carvone analogues: olfactory proper...
2000-09-01 [Eur. J. Med. Chem. 35(9) , 797-803, (2000)] |