Solid-state surface acidity and pH-stability profiles of amorphous quinapril hydrochloride and silicate formulations.
Shumet A Hailu, Robin H Bogner
Index: J. Pharm. Sci. 99(6) , 2786-99, (2010)
Full Text: HTML
Abstract
To determine the surface acidity and stability profiles of quinapril hydrochloride (QHCl) coground with silicates, solid-state equivalent pH (pHeq) of amorphous samples was measured by diffuse reflectance spectroscopy using pH indicator probes. Calibration curves for pH indicators were developed in buffer solutions. Amorphous samples with and without pH indicators were prepared by cryo-grinding. Different pH grades of silicates and various QHCl/silicate ratios were used to make amorphous samples over a range of surface acidity. Diffuse reflectance spectra of amorphous samples were measured and pHeq was determined using the calibration curves of pH indicators developed in solution. Suspension pH of amorphous samples was also measured. The chemical stability of coground amorphous samples was assessed at 40 degrees C and 0% or 48% RH. The chemical stability of neat amorphous quinapril lyophilized from solutions over a range of pH was also assessed at 40 degrees C/0% RH and the reconstituted pH-stability profile of lyophiles was determined. For all silicate and QHCl/silicate amorphous samples, the same pH rank order was obtained based on pHeq and suspension pH. However, the pHeq was significantly lower than the corresponding suspension pH. Discrepancies between pH-stability profiles based on the pHeq and the suspension pH were observed. In general, the pHeq- and reconstituted pH-stability profiles were essentially identical, but the suspension pH-stability profile deviated from the reconstituted pH-stability profile by 2-3 pH units. The results indicate that solid-state surface acidity measurement provides a more accurate prediction of the effective surface acidity of amorphous formulations than the suspension pH. In conclusion, solid-state surface acidity measurement of excipients and solid formulations using pH indicator probes as surrogates can be used to determine the ionization state of the drug and to predict the chemical stability profile of the drug in actual solid formulations.(c) 2010 Wiley-Liss, Inc. and the American Pharmacists Association
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
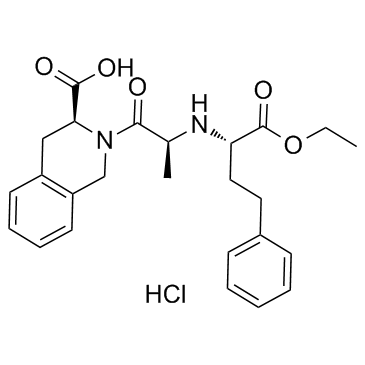 |
Quinapril hydrochloride
CAS:82586-55-8 |
C25H31ClN2O5 |
|
Determination of losartan potassium, quinapril hydrochloride...
2013-01-01 [Acta Pol. Pharm. 70(6) , 967-76, (2013)] |
|
Hepatic, intestinal, renal, and plasma hydrolysis of prodrug...
2014-09-01 [Drug Metab. Dispos. 42(9) , 1522-31, (2014)] |
|
The impact of lipoic acid on endothelial function and protei...
2012-06-01 [J. Cardiovasc. Pharmacol. Ther. 17(2) , 139-45, (2012)] |
|
Increased renoprotection with ACE inhibitor plus aldosterone...
2009-12-01 [Nephrol. Dial. Transplant. 24(12) , 3640-51, (2009)] |
|
Effects of ACE inhibitors on cardiac angiotensin II and aldo...
2010-11-01 [Am. J. Hypertens. 23(11) , 1179-82, (2010)] |