The reaction of butylated hydroxyanisole and its metabolites with some arylamines: investigations of product mutagenicity.
W H Kalus, R Münzner, W G Filby
Index: Environ. Health Perspect. 102(1) , 96-9, (1994)
Full Text: HTML
Abstract
We examined t-butylhydroquinone (t-BHQ) and t-butylquinone (t-BuQ), two of the major microsomal metabolites of the synthetic antioxidant butylated hydroxyanisole (BHA), for their ability to react with the xenobiotic arylamines aniline and N-methylaniline. A number of substances were isolated by thin-layer chromatography. The main products were quantitatively evaluated and their structures assigned. BHA and t-BHQ yielded reaction products with anilines only in the presence of an oxidant such as iodate (KIO3). We used the Salmonella/microsome mutagenicity assay to test the new compounds for mutagenic activity. The reaction products gave no evidence of mutagenicity in the S. typhimurium strains TA98 and TA100, with or without metabolic activation. In some instances the substituted quinone products are less toxic than t-BuQ alone.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
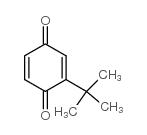 |
2-tert-Butyl-1,4-benzoquinone
CAS:3602-55-9 |
C10H12O2 |
|
Reduction of cytotoxic p-quinone metabolites of tert-butylhy...
2012-01-01 [Drug Metab. Pharmacokinet. 27(5) , 553-8, (2012)] |
|
Paradoxical cytotoxicity of tert-butylhydroquinone in vitro:...
2012-09-01 [Arch. Toxicol. 86(9) , 1481-7, (2012)] |
|
Irreversible inhibition of rat hepatic glutathione S-transfe...
1989-01-01 [Chem. Biol. Interact. 71(4) , 381-92, (1989)] |
|
Initiating activity of 3-tert-butyl-4-hydroxyanisole (3-BHA)...
1990-11-01 [Carcinogenesis 11(11) , 1985-8, (1990)] |
|
Formation of the semiquinone anion radical from tert-butylqu...
1992-09-01 [Toxicology 74(2-3) , 127-33, (1992)] |