On the isomerization of ent-kaurenic acid.
Julio Rojas, Rosa Aparicio, Thayded Villasmil, Alexis Peña, Alfredo Usubillaga
Index: Nat. Prod. Commun. 6(7) , 935-8, (2011)
Full Text: HTML
Abstract
Kaurenic acid (1a) is a tetracyclic diterpene that has an exocyclic double bond at delta16. Isokaurenic acid (2a) has an endocyclic delta15double bond. This compound has been isolated from Espeletia tenore (Espeletinae), a resinous plant from the Venezuelan Andes, but its occurrence is rare. In order to obtain a larger amount of 2a, the isomerization of la, which is easily obtained from other Espeletinae, was tried. Kaurenic acid methyl ester (1b) was treated with dil. HCl in CH3Cl/EtOH, after 6 h under reflux a yield of 41.5% isokaurenic acid methyl ester (2b) was obtained but 35.7% 16alpha-ethoxy-kauran-19-oic acid methyl ester (3b) had formed as a byproduct. Treating 1b with CF3COOH in refluxing CH2Cl2 permitted to obtain a yield of 66.6% of 2b in 4 h and only traces of 16alpha-hydroxy-kauran-19-oic acid methyl ester (3a) as a byproduct. Both isomers were separated on a silica gel column impregnated with 20% AgNO3. Treating 2b with KOH in refluxing DMSO yielded pure isokaurenic acid, no back isomerization was observed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
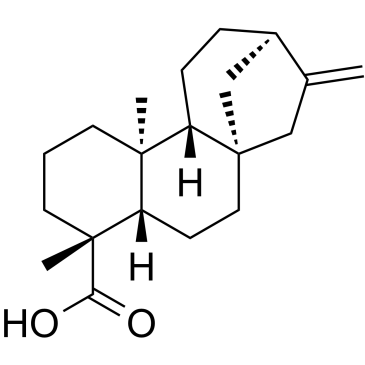 |
Kaurenoic acid
CAS:6730-83-2 |
C20H30O2 |
|
Development and validation of two methods based on high-perf...
2011-04-01 [J. Sep. Sci. 34(7) , 740-8, (2011)] |
|
Anticonvulsant effect of kaurenoic acid isolated from the ro...
2013-08-01 [Pharmacol. Biochem. Behav. 109 , 38-43, (2013)] |
|
Biosynthesis of uterotonic diterpenes from Montanoa tomentos...
2009-12-15 [J. Plant Physiol. 166(18) , 1961-7, (2009)] |
|
Isolation of two bioactive diterpenic acids from Copaifera g...
2010-01-01 [Phytochem. Anal. 21(6) , 539-43, (2010)] |
|
Hypoglycaemic effects of tea extracts and ent-kaurenoic acid...
2010-11-01 [Nat. Prod. Res. 24(18) , 1771-82, (2010)] |