| Structure | Name/CAS No. | Articles |
|---|---|---|
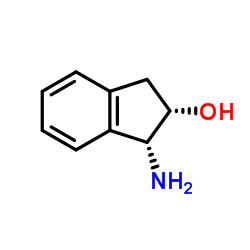 |
(1R,2S)-1-Amino-2,3-dihydro-1H-inden-2-ol
CAS:136030-00-7 |
W J Thompson, P M Fitzgerald, M K Holloway, E A Emini, P L Darke, B M McKeever, W A Schleif, J C Quintero, J A Zugay, T J Tucker
Index: J. Med. Chem. 35 , 1685, (1992)
Full Text: HTML
By tethering of a polar hydrophilic group to the P1 or P1' substituent of a Phe-based hydroxyethylene isostere, the antiviral potency of a series of HIV protease inhibitors was improved. The optimum enhancement of anti-HIV activity was observed with the 4-morpholinylethoxy substituent. The substituent effect is consistent with a model derived from inhibitor docked in the crystal structure of the native enzyme. An X-ray crystal structure of the inhibited enzyme determined to 2.25 A verifies the modeling predictions.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
(1R,2S)-1-Amino-2,3-dihydro-1H-inden-2-ol
CAS:136030-00-7 |
C9H11NO |
|
A series of potent HIV-1 protease inhibitors containing a hy...
1992-07-10 [J. Med. Chem. 35 , 2525, (1992)] |
|
HIV-1 protease inhibitors based on hydroxyethylene dipeptide...
1992-05-15 [J. Med. Chem. 35 , 1702, (1992)] |
|
Inhibitors of the protease from human immunodeficiency virus...
1993-04-16 [J. Med. Chem. 36 , 941, (1993)] |
|
D'Aniello, F. et al.
[J. Org. Chem. 59 , 3762, (1994)] |
|
Dorsey, B.D. et al.
[Tetrahedron Lett. 34 , 1851, (1993)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2024 ChemSrc All Rights Reserved