| Structure | Name/CAS No. | Articles |
|---|---|---|
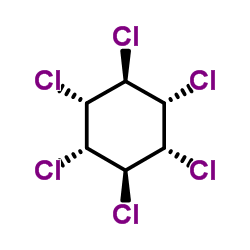 |
γ-lindane
CAS:58-89-9 |
|
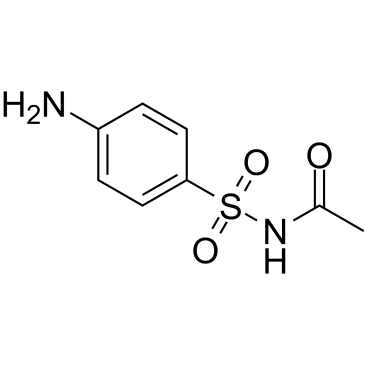 |
sulfacetamide
CAS:144-80-9 |
|
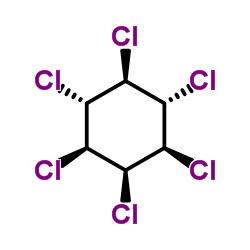 |
δ-Hexachlorocyclohexane
CAS:319-86-8 |
|
 |
Argipressin
CAS:113-79-1 |
|
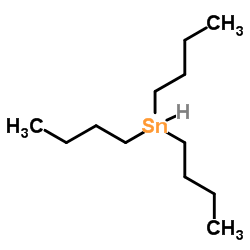 |
Tributyltin hydride
CAS:688-73-3 |
|
 |
GHRP-6 Acetate
CAS:87616-84-0 |