| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
3-Aminopropyltriethoxysilane
CAS:919-30-2 |
|
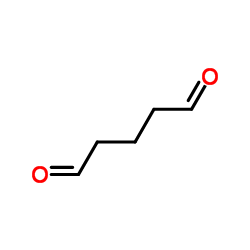 |
glutaraldehyde
CAS:111-30-8 |
|
 |
Polyethylene-polypropylene glycol
CAS:9003-11-6 |