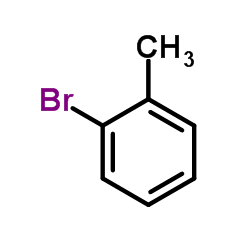
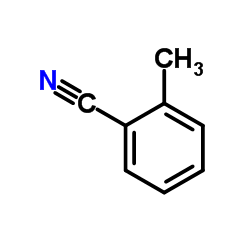
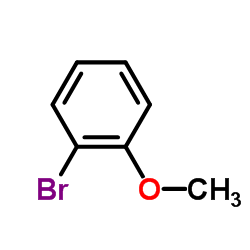
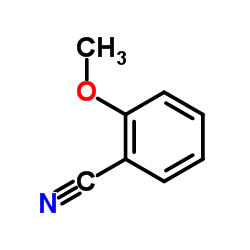
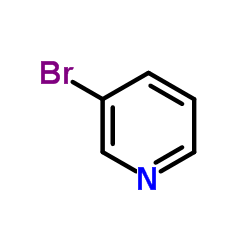
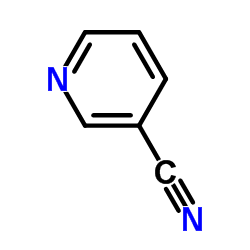
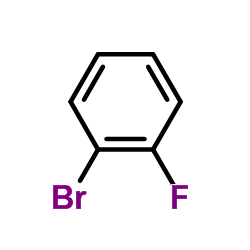
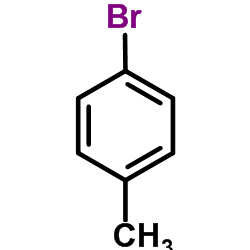
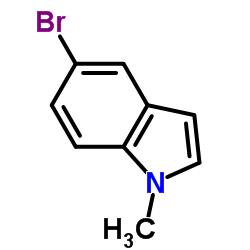
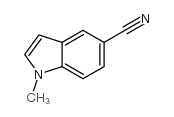
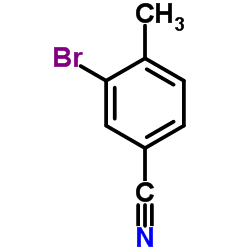
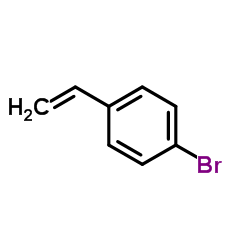
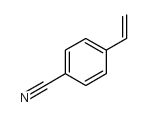
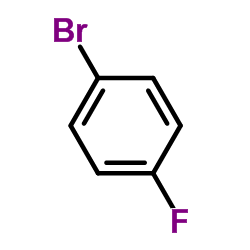
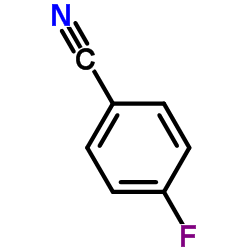
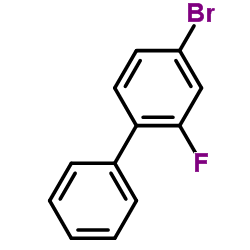
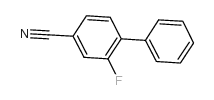
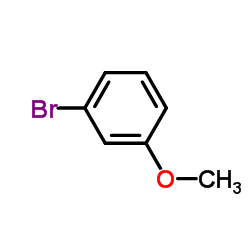
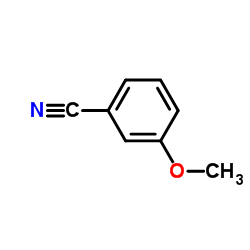
|
~47% |
|
~78% |
|
~95% |
|
~76% |
|
~63% |
|
~77% |
|
~86% |
|
~72% |
|
~83% |
|
~70% |
|
~68% |
|
~69% |
|
~73% |
|
~71% |
|
~80% |
|
~78% |
|
~82% |
|
~83% |
|
~78% |
|
~88% |
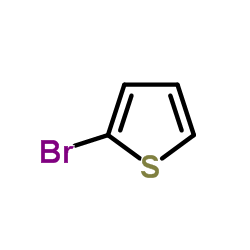
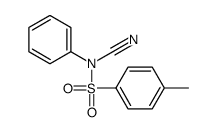
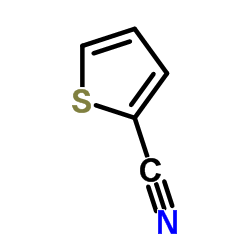
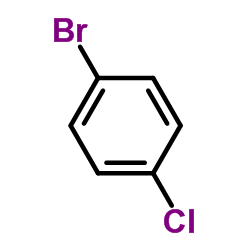
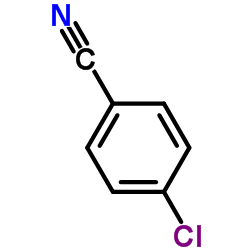
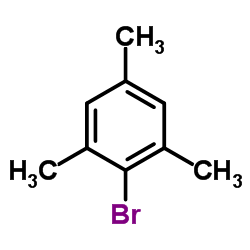
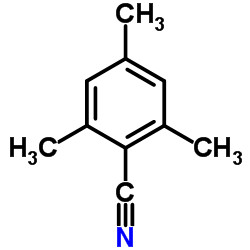
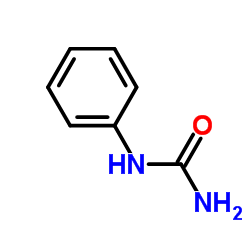
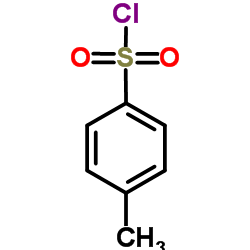
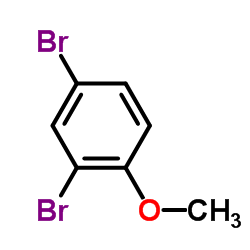
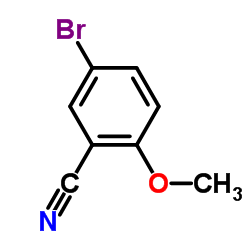


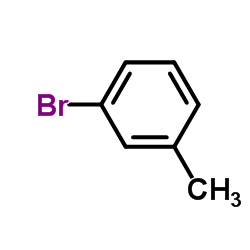
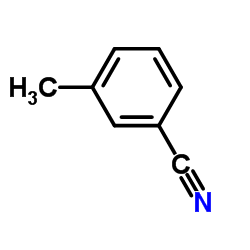

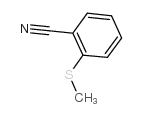
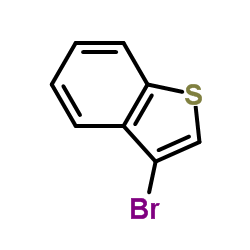
![Benzo[b]thiophene-3-carbonitrile Structure](https://image.chemsrc.com/caspic/467/24434-84-2.png)