[Which analgesic after dextropropoxyphene withdrawal? A survey in a sample of general practitioners in southwest of France].
Serge Bismuth, Eng Laing Leng, Stéphane Oustric, Jean-Louis Montastruc, Maryse Lapeyre-Mestre
Index: Therapie. 66(1) , 25-8, (2011)
Full Text: HTML
Abstract
Following the announcement of the future withdrawal in Europe of drugs containing dextropropoxyphene-paracetamol (DXP-P), we performed a postal survey in a randomly selected sample of 350 general practitioners (GP) from the Midi-Pyrénées area (2.6 million inhabitants) in order to investigate which drug (s) they are willing to prescribe in anticipation of the announced withdrawal. Most of GP prescribed DXP-P in acute and chronic pain. In acute pain, GP would switch to codeine-paracetamol (59.1%) or tramadol alone or associated with paracetamol (79%), whereas they would switch to high dose paracetamol (54.7%) and tramadol alone or associated with paracetamol (74.6%) in chronic pain. Switching to other level 2 analgesic drugs after the withdrawal of dextropropoxyphene should be closely monitored because the safety profile of other drugs.© 2011 Société Française de Pharmacologie et de Thérapeutique.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
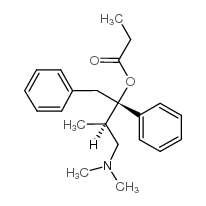 |
dextropropoxyphene
CAS:469-62-5 |
C22H29NO2 |
|
Clinical toxicology in Edinburgh, two centuries of progress.
2013-07-01 [Clin. Toxicol. (Phila.) 51(6) , 509-14, (2013)] |
|
Diplopia with dextropropoxyphene withdrawal.
2013-02-01 [Gen. Hosp. Psychiatry 35(1) , 100-1, (2013)] |
|
Urine drug testing of chronic pain patients. V. Prevalence o...
2013-01-01 [J. Anal. Toxicol. 37(1) , 1-4, (2013)] |
|
Physician perspective on propoxyphene as a potentially inapp...
2011-07-01 [South. Med. J. 104(7) , 533-9, (2011)] |
|
Synthetic opioids and arrhythmia risk: a new paradigm?
2012-09-01 [Expert Opin. Pharmacother. 13(13) , 1825-7, (2012)] |