A ring contraction strategy toward a diastereoselective total synthesis of (+)-bakkenolide A.
Vânia M T Carneiro, Helena M C Ferraz, Tiago O Vieira, Eloisa E Ishikawa, Luiz F Silva
Index: J. Org. Chem. 75(9) , 2877-82, (2010)
Full Text: HTML
Abstract
A diastereoselective route to (+)-bakkenolide A is presented from the readily available optically active Wieland-Miescher ketone. This novel synthesis of this sesquiterpene lactone features the following as key stereoselective transformations: (i) the ring contraction reaction of a octalone mediated by thallium(III) nitrate (TTN); (ii) a hydrogenation to create the cis-fused junction; and (iii) the formation of the C7 quaternary center through an enolate intermediate. Furthermore, during this work, the absolute configuration of a trinorsesquiterpene isolated from Senecio humillimus was assigned.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
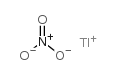 |
Nitric acid,thallium(1+) salt (1:1)
CAS:10102-45-1 |
NO3Tl |
|
Simultaneous determination of plant growth regulators 1-naph...
2012-01-01 [Phytochem. Anal. 23(3) , 214-21, (2012)] |
|
Thallium exists in opioid poisoned patients.
2015-01-01 [Daru 23 , 39, (2015)] |
|
Prokaryotic NavMs channel as a structural and functional mod...
2014-06-10 [Proc. Natl. Acad. Sci. U. S. A. 111(23) , 8428-33, (2014)] |
|
Tl(I) and Tl(III) alter the expression of EGF-dependent sign...
2015-08-01 [J. Appl. Toxicol. 35 , 952-69, (2015)] |
|
[Action of the salts of univalent thallium on the liver mito...
1984-01-01 [Zh. Evol. Biokhim. Fiziol. 20(4) , 353-5, (1984)] |