| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
D-Fructose-1,6-diphosphate dicalcium salt
CAS:6055-82-9 |
|
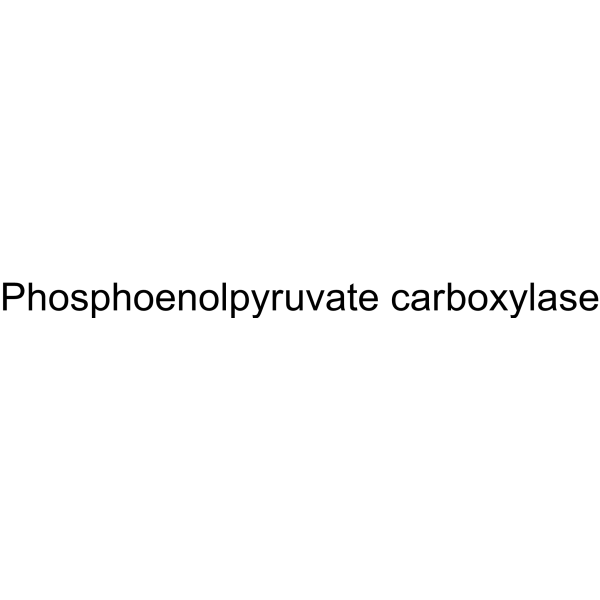 |
Phosphoenolpyruvate carboxylase
CAS:9067-77-0 |