Synthesis of tri- or tetrasubstituted pyrimidine derivatives through the [5+1] annulation of enamidines with either N,N-dimethylformamide dialkyl acetals or orthoesters and their application in a ring transformation of pyrimidines to pyrido[2,3-d]pyrimidin-5-one derivatives.
Toshiaki Sasada, Youichi Aoki, Reiko Ikeda, Norio Sakai, Takeo Konakahara
Index: Chemistry 17(34) , 9385-94, (2011)
Full Text: HTML
Abstract
The [5+1] annulation of enamidines, which were prepared from functionalized silanes, organolithium compounds and two nitriles, with N,N-dimethylformamide dialkyl acetals as the C1 unit is described, leading to the synthesis of tri- and tetrasubstituted pyrimidine derivatives under catalyst- and solvent-free reaction conditions. Furthermore, the [5+1] annulation of enamidines by using orthoesters as the C1 unit is described, in which catalytic amounts of ZnBr(2) catalyze the annulation to produce polysubstituted pyrimidines under toluene or xylene reflux conditions. Moreover, the combination of a reductive ring-opening reaction with [Mo(CO)(6)] and a subsequent intramolecular cyclization with tBuOK effectively causes a skeletal transformation from the pyrimidines containing an isoxazolyl and an ethoxy substituent to form pyrido[2,3-d]pyrimidin-5-one frameworks in excellent yield.Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
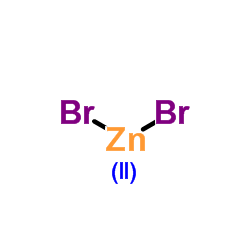 |
Zinc bromide
CAS:7699-45-8 |
Br2Zn |
|
Asymmetric Rh-catalyzed intermolecular hydroacylation of 1,5...
2009-10-01 [Chem. Pharm. Bull. 57(10) , 1158-60, (2009)] |
|
Palladium-catalyzed cross-coupling of cyclopropylmagnesium b...
2010-10-01 [J. Org. Chem. 75(19) , 6677-80, (2010)] |
|
Stereoselective glycosylations using benzoylated glucosyl ha...
2008-06-09 [Carbohydr. Res. 343(8) , 1297-308, (2008)] |
|
[Halogenated anesthetic vaporizers: importance of maintenanc...
1998-01-01 [Ann. Fr. Anesth. Reanim. 17(7) , 747-9, (1998)] |
|
alpha,alpha-gem-Difluorination of alpha-(alkylthio)acetophen...
2000-07-01 [Chem. Pharm. Bull. 48(7) , 1097-100, (2000)] |