Effect of the reactant mixing sequence on the chymotrypsin inhibition assay.
K S LIU, P Markakis
Index: Analyst 115(8) , 1143-4, (1990)
Full Text: HTML
Abstract
In assaying chymotrypsin inhibition by the soybean Bowman-Birk inhibitor, two sequences of mixing the reactants were tried: adding the substrate last (s-last test) or adding the enzyme last (e-last test). The inhibition values obtained from the s-last test were either equal to or lower than those from the e-last test, depending on the pre-mix pH and pre-incubation time, while the values from the e-last test were independent of these conditions. The differences between the two tests were largest at a pre-mix pH of 4.0 or 8.5 and zero near pH 7.0. Abruptly changing the pre-mix pH from 4.0 to 7.0 brought the values of the two tests closer. These observations suggest a reversible, yet limited, hydrolysis of the inhibitor by the very enzyme it inhibits and indicate the greater reliability of the e-last test over the s-last test, paralleling a similar previous finding on the trypsin inhibition assay.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
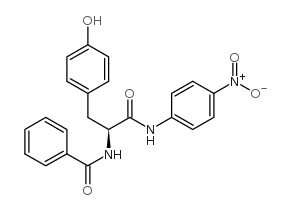 |
Bz-Tyr-pNA
CAS:6154-45-6 |
C22H19N3O5 |
|
Preliminary characterisation of digestive proteases of the g...
2001-03-15 [Insect Biochem. Mol. Biol. 31 , 415-423, (2001)] |
|
Chymotrypsin immobilization on epoxy monolithic silica colum...
2007-11-01 [J. Sep. Sci. 30 , 3069-3076, (2007)] |
|
Purification and characterization of a serine carboxypeptida...
2004-03-15 [Int. J. Food Microbiol. 91 , 245-252, (2004)] |
|
The chymotrypsin inhibitor carbobenzyloxy-leucine-tyrosine-c...
1991-09-15 [J. Immunol. 147(6) , 1912-9, (1991)] |
|
Activation and stabilization of chymotrypsin in microdomains...
1998-06-02 [Bioorg. Med. Chem. Lett. 8(11) , 1331-6, (1998)] |