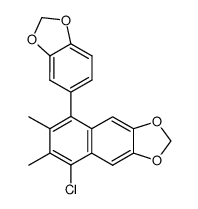
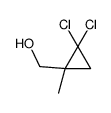
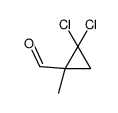
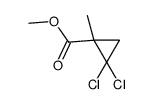
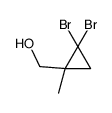
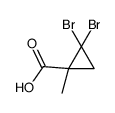
|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~% |
|
~82% |
|
~91% |

![Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(8H)-one,9-(1,3-benzodioxol-5-yl) Structure](https://image.chemsrc.com/caspic/251/27792-97-8.png)