| Structure | Name/CAS No. | Articles |
|---|---|---|
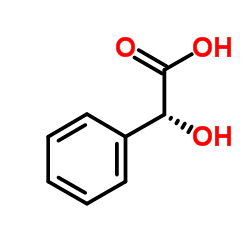 |
(S)-(+)-Mandelic acid
CAS:17199-29-0 |
|
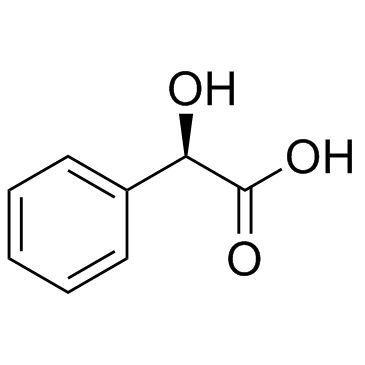 |
Mandelic acid
CAS:611-71-2 |
|
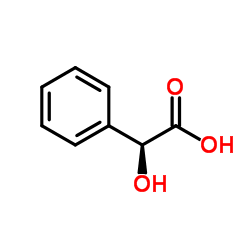 |
DL-Mandelic acid
CAS:90-64-2 |