| Structure | Name/CAS No. | Articles |
|---|---|---|
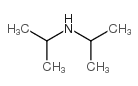 |
Diisopropylamine
CAS:108-18-9 |
|
 |
Lithium diisopropylamide
CAS:4111-54-0 |