Application of the guanidine-acylguanidine bioisosteric approach to argininamide-type NPY Y₂ receptor antagonists.
Nikola Pluym, Albert Brennauer, Max Keller, Ralf Ziemek, Nathalie Pop, Günther Bernhardt, Armin Buschauer
Index: ChemMedChem 6(9) , 1727-38, (2011)
Full Text: HTML
Abstract
Strongly basic groups such as guanidine moieties are crucial structural elements, but they compromise the drug-likeness of numerous biologically active compounds, including ligands of G-protein-coupled receptors (GPCRs). As part of a project focused on the search for guanidine bioisosteres, argininamide-type neuropeptide Y (NPY) Y₂ receptor (Y₂R) antagonists related to BIIE0246 were synthesized. Starting from ornithine derivatives, N(G) -acylated argininamides were obtained by guanidinylation with tailor-made mono-Boc-protected N-acyl-S-methylisothioureas. The compounds were investigated for Y₂R antagonism (calcium assays), Y₂R affinity, and NPY receptor subtype selectivity (flow cytometric binding assays). Most of the N(G) -substituted (S)-argininamides showed Y₂R antagonistic activities and binding affinities similar to those of the parent compound, whereas N(G)-acylated or -carbamoylated analogues with a terminal amine were superior (Y₂R: K(i) and K(B) values in the low nanomolar range). This demonstrates that the basicity of the compounds, although 4-5 orders of magnitude lower than that of guanidines, is sufficient to form key interactions with acidic amino acids of the Y₂R. The acylguanidines bind with high affinity and selectivity to Y₂R over the Y₁, Y₄, and Y₅ receptors. As derivatization of the amino group is tolerated, these compounds can be considered building blocks for the preparation of versatile fluorescent and radiolabeled pharmacological tools for in vitro studies of the Y₂R. The results support the concept of bioisosteric guanidine-acylguanidine exchange as a broadly applicable approach to retain pharmacological activity despite decreased basicity.Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
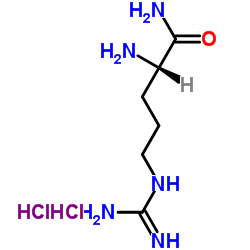 |
H-Arg-NH2.2HCl
CAS:14975-30-5 |
C6H17Cl2N5O |
|
Immobilization of trypsin in organic and aqueous media for e...
2015-01-01 [BMC Biotechnol. 15 , 77, (2015)] |
|
Energetic basis of molecular recognition in a DNA aptamer.
2007-03-01 [Biophys. Chem. 126(1-3) , 165-75, (2007)] |
|
N(G)-Acyl-argininamides as NPY Y(1) receptor antagonists: In...
2010-09-01 [Bioorg. Med. Chem. 18(17) , 6292-304, (2010)] |
|
Structural features of the L-argininamide-binding DNA aptame...
2006-10-15 [Anal. Chem. 78(20) , 7259-66, (2006)] |
|
Red-fluorescent argininamide-type NPY Y1 receptor antagonist...
2011-05-01 [Bioorg. Med. Chem. 19(9) , 2859-78, (2011)] |