Novel benzyl-substituted N-heterocyclic carbene-silver acetate complexes: synthesis, cytotoxicity and antibacterial studies.
Siddappa Patil, Anthony Deally, Brendan Gleeson, Helge Müller-Bunz, Francesca Paradisi, Matthias Tacke
Index: Metallomics 3(1) , 74-88, (2011)
Full Text: HTML
Abstract
From the reaction of 1-methylimidazole (1a), 4,5-dichloro-1H-imidazole (1b(I)) and 1-methylbenzimidazole (1c) with p-cyanobenzyl bromide (2a), non-symmetrically substituted N-heterocyclic carbene (NHC) [(3a-c)] precursors, 5,6-dimethyl-1H-benzimidazole (1d) and 4,5-diphenyl-1H-imidazole (1e) with p-cyanobenzyl bromide (2a) and benzyl bromide (2b), symmetrically substituted N-heterocyclic carbene (NHC) [(3d-f)] precursors were synthesised. These NHC-precursors were then reacted with silver(i) acetate to yield the NHC-silver complexes (1-methyl-3-(4-cyanobenzyl)imidazole-2-ylidene)silver(i)acetate (4a), (4,5-dichloro-1-(4-cyanobenzyl)-3-methyl)imidazole-2-ylidene)silver(i)acetate (4b), (1-methyl-3-(4-cyanobenzyl)benzimidazole-2-ylidene)silver(i)acetate (4c), (1,3-bis(4-cyanobenzyl)5,6-dimethylbenzimidazole-2-ylidene) silver(i) acetate (4d), (1,3-dibenzyl-5,6-dimethylbenzimidazole-2-ylidene) silver(i) acetate (4e) and (1,3-dibenzyl-4,5-diphenylimidazol-2-ylidene) silver(i) acetate (4f) respectively. Three NHC-precursors 3c-e and four NHC-silver complexes 4b and 4d-f were characterised by single crystal X-ray diffraction. Preliminary in vitro antibacterial activity of the NHC-precursors and NHC-silver complexes was investigated against Gram-positive bacteria Staphylococcus aureus, and Gram-negative bacteria Escherichia coli using the qualitative Kirby-Bauer disk-diffusion method. NHC-silver complexes have shown very high antibacterial activity compared to the NHC-precursors. All six NHC-silver complexes were tested for their cytotoxicity through MTT based in vitro tests on the human renal-cancer cell line Caki-1 in order to determine their IC₅₀ values. NHC-silver complexes 4a-f were found to have IC₅₀ values of 6.2 (±1.0), 7.7 (±1.6), 1.2 (±0.6), 10.8 (±1.9), 24.2 (±1.8) and 13.6 (±1.0) μM, respectively. These values represent improved cytotoxicity against Caki-1, most notably for 4c, which is a three times more cytotoxic than cisplatin (IC₅₀ value = 3.3 μM) itself.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
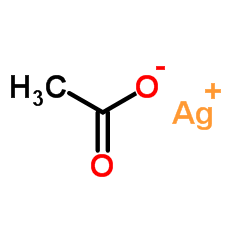 |
Silver acetate
CAS:563-63-3 |
C2H3AgO2 |
|
Silver acetate for smoking cessation.
2012-01-01 [Cochrane Database Syst. Rev. 9 , CD000191, (2012)] |
|
Distribution of silver in rats following 28 days of repeated...
2011-01-01 [Part. Fibre Toxicol. 8 , 18, (2011)] |
|
Silver acetate catalyzed hydroamination of 1-(2-(sulfonylami...
2012-09-07 [J. Org. Chem. 77(17) , 7166-75, (2012)] |
|
Subacute oral toxicity investigation of nanoparticulate and ...
2012-04-01 [Arch. Toxicol. 86(4) , 543-51, (2012)] |
|
Synthesis of beta-, gamma-, and delta-lactams via Pd(II)-cat...
2008-10-29 [J. Am. Chem. Soc. 130(43) , 14058-9, (2008)] |