| Structure | Name/CAS No. | Articles |
|---|---|---|
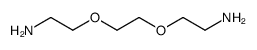 |
Polyethylene glycol diamine
CAS:24991-53-5 |
|
 |
Ac-Lys(Ac)-D-Ala-D-Ala-OH
CAS:24570-39-6 |