Entropy-driven complex formation of malvidin-3-O-glucoside with common polyphenols in ethanol-water binary solutions.
Sándor Kunsági-Máté, Erika Ortmann, László Kollár, Martin Pour Nikfardjam
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 70(4) , 860-5, (2008)
Full Text: HTML
Abstract
The complex formation of malvidin-3-O-glucoside with several polyphenols, the so-called "copigmentation" phenomenon, was studied in aqueous solutions. To simulate the copigmentation process during fermentation, the stability of the formed complexes was examined in dependence of the ethanol content of the aqueous solution. Results indicate that stronger and larger complexes are formed, when the ethanol content exceeds a critical margin of 8vol.% However, the size of complexes of malvidin/procyanidin and malvidin/epicatechin is drastically reduced above this critical concentration. Fluorescence lifetime and solvent relaxation measurements give insight into the particular processes at molecular level and will help us comprehend the first important steps during winemaking in order to recommend an optimized winemaking technology to ensure extraordinary colour stability in red wines.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
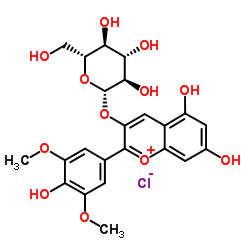 |
Malvidin-3-O-glucoside chloride
CAS:7228-78-6 |
C23H25ClO12 |
|
The identification of degradation products and degradation p...
2013-12-01 [Food Chem. 141(3) , 3260-7, (2013)] |
|
Malvidin-3-glucoside protects endothelial cells up-regulatin...
2012-09-30 [Chem. Biol. Interact. 199(3) , 192-200, (2012)] |
|
Comprehensive colorimetric study of anthocyanic copigmentati...
2012-03-21 [J. Agric. Food Chem. 60(11) , 2896-905, (2012)] |
|
Induction of apoptosis and inhibition of invasion in human h...
2009-08-01 [Ann. N. Y. Acad. Sci. 1171 , 137-48, (2009)] |
|
Principal components of phenolics to characterize redVinho V...
2008-01-01 [Talanta 75(5) , 1190-202, (2008)] |