Efficient Cu-catalyzed base-free C-S coupling under conventional and microwave heating. A simple access to S-heterocycles and sulfides.
Silvia M Soria-Castro, Alicia B Peñéñory
Index: Beilstein J. Org. Chem. 9 , 467-75, (2013)
Full Text: HTML
Abstract
S-aryl thioacetates can be prepared by reaction of inexpensive potassium thioacetate with both electron-rich and electron-poor aryl iodides under a base-free copper/ligand catalytic system. CuI as copper source affords S-aryl thioacetates in good to excellent yields, by using 1,10-phenanthroline as a ligand in toluene at 100 °C after 24 h. Under microwave irradiation the time was drastically reduced to 2 h. Both procedures are simple and involve a low-cost catalytic system. This methodology was also applied to the "one-pot" synthesis of target heterocycles, such as 3H-benzo[c][1,2]dithiol-3-one and 2-methylbenzothiazole, alkyl aryl sulfides, diaryl disulfides and asymmetric diaryl sulfides in good yields.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
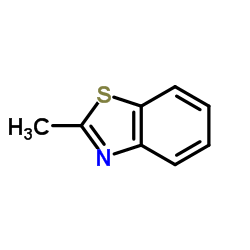 |
2-Methylbenzothiazole
CAS:120-75-2 |
C8H7NS |
|
Benzothiazole, benzotriazole, and their derivates in clothin...
2015-04-01 [Environ. Sci. Pollut. Res. Int. 22(8) , 5842-9, (2015)] |
|
Simultaneous determination of benzothiazoles, benzotriazoles...
2010-01-01 [J. Chromatogr. A. 1338 , 164-73, (2014)] |
|
A Programmed DNA Marker Based on Bis(4-ethynyl-1,8-naphthali...
2015-11-09 [Chemistry 21 , 16623-30, (2015)] |
|
Two-cationic 2-methylbenzothiazole derivatives as green ligh...
2015-07-01 [Colloid Polym. Sci. 293 , 1865-1876, (2015)] |
|
[Zh. Org. Khim. 28 , 2159, (1992)] |