| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Disilver(1+) carbonate
CAS:534-16-7 |
|
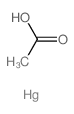 |
mercuric acetate
CAS:1600-27-7 |