| Structure | Name/CAS No. | Articles |
|---|---|---|
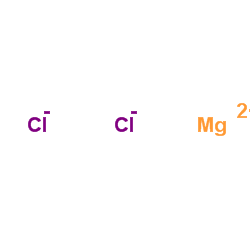 |
Magnesium choride
CAS:7786-30-3 |
|
 |
Cupric chloride
CAS:7447-39-4 |
|
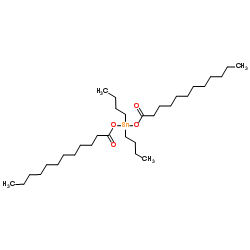 |
Dibutyltin dillaurate
CAS:77-58-7 |
|
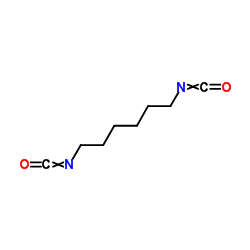 |
1,6-Diisocyanatohexane
CAS:822-06-0 |