Quality of Life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores.
Alexandra L Quittner, Anne E O'Donnell, Matthias A Salathe, Sandra A Lewis, Xiaoming Li, A Bruce Montgomery, Thomas G O'Riordan, Alan F Barker
Index: Thorax 70(1) , 12-20, (2015)
Full Text: HTML
Abstract
The Quality of Life-Bronchiectasis (QOL-B), a self-administered, patient-reported outcome measure assessing symptoms, functioning and health-related quality of life for patients with non-cystic fibrosis (CF) bronchiectasis, contains 37 items on 8 scales (Respiratory Symptoms, Physical, Role, Emotional and Social Functioning, Vitality, Health Perceptions and Treatment Burden).Psychometric analyses of QOL-B V.3.0 used data from two double-blind, multicentre, randomised, placebo-controlled, phase III trials of aztreonam for inhalation solution (AZLI) in 542 patients with non-CF bronchiectasis and Gram-negative endobronchial infection.Excellent internal consistency (Cronbach's α ≥0.70) and 2-week test-retest reliability (intraclass correlation coefficients ≥0.72) were demonstrated for each scale. Convergent validity with 6 min walk test was observed for Physical and Role Functioning scores. No floor or ceiling effects (baseline scores of 0 or 100) were found for the Respiratory Symptoms scale (primary endpoint of trials). Baseline Respiratory Symptoms scores discriminated between patients based on baseline FEV₁% predicted in only one trial. The minimal important difference score for the Respiratory Symptoms scale was 8.0 points. AZLI did not show efficacy in the two phase III trials. QOL-B responsivity to treatment was assessed by examining changes from baseline QOL-B scores at study visits at which protocol-defined pulmonary exacerbations were reported. Mean Respiratory Symptoms scores decreased 14.0 and 14.2 points from baseline for placebo-treated and AZLI-treated patients with exacerbations, indicating that worsening respiratory symptoms were reflected in clinically meaningful changes in QOL-B scores.Previously established content validity, reliability and responsivity of the QOL-B are confirmed by this final validation study. The QOL-B is available for use in clinical trials and routine clinical practice.Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
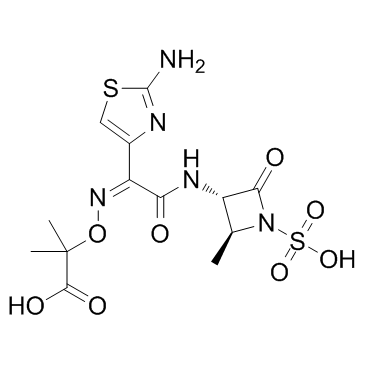 |
Aztreonam
CAS:78110-38-0 |
C13H17N5O8S2 |
|
Tolerability of aztreonam and carbapenems in patients with I...
2015-04-01 [J. Allergy Clin. Immunol. 135(4) , 972-6, (2015)] |
|
In vitro antibacterial activity of AZD0914, a new spiropyrim...
2015-01-01 [Antimicrob. Agents Chemother. 59(1) , 467-74, (2014)] |
|
An in vitro deletion in ribE encoding lumazine synthase cont...
2014-12-01 [Antimicrob. Agents Chemother. 58(12) , 7225-33, (2014)] |
|
LTX-109 is a novel agent for nasal decolonization of methici...
2015-01-01 [Antimicrob. Agents Chemother. 59(1) , 145-51, (2014)] |
|
An outbreak of blaOXA-51-like- and blaOXA-66-positive Acinet...
2014-11-01 [J. Med. Microbiol. 63(Pt 11) , 1517-23, (2014)] |