[The compatibility of ronidazol in racing pigeons in a controlled clinical study].
M E Krautwald-Junghanns, R Streubel, V Schmidt, A Richter, H J Zumbusch, N Lohmann, A Daugschies
Index: DTW Dtsch. Tierarztl. Wochenschr. 111(6) , 231-6, (2004)
Full Text: HTML
Abstract
Ronidazol is often used in racing pigeons for the treatment of Trichomonas infections and diseases. Therefore, in this study, the compatibility of the drug was examined by oral application over 7 days. For this purpose a randomized blind study was performed using four different groups (control group, 10 mg = therapy-group, 20 mg = double-dose-group and 40 mg = high-dose-group) of pigeons (Columba livia f. domestica) with 6 male and 6 female birds each. All birds were clinically healthy and between 6 and 12 weeks of age. The application of ronidazol at a dose of 10 mg/racing pigeon did show no side-effect within the duration of the study, e.g. no influence could be seen on clinical, haematological, blood-chemical and pathological parameters. Low-to middle-grade clinical alterations of the gastro-intestinal tract occurred in the high-dose group at day 6 and 7 of the application of the drug. Therefore a fourfold overdosing of ronidazol should be avoided.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
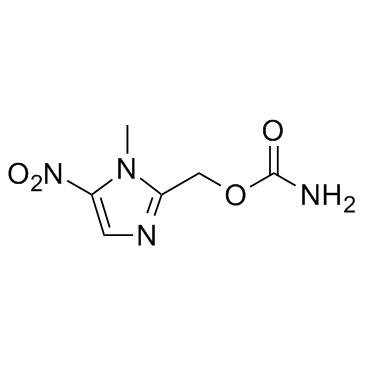 |
Ronidazole
CAS:7681-76-7 |
C6H8N4O4 |
|
Determination of nitroimidazole residues in aquaculture tiss...
2014-06-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 960 , 105-15, (2014)] |
|
Simultaneous determination of 15 nitroimidazoles in cosmetic...
2014-01-01 [J. AOAC Int. 97(6) , 1538-45, (2015)] |
|
Tritrichomonas foetus infection in cats with diarrhoea in a ...
2009-02-01 [J. Feline Med. Surg. 11(2) , 131-4, (2009)] |
|
Development of a rapid method for the determination and conf...
2012-05-01 [J. Pharm. Biomed. Anal. 64-65 , 40-8, (2012)] |
|
Ronidazole pharmacokinetics after intravenous and oral immed...
2011-04-01 [J. Feline Med. Surg. 13(4) , 244-50, (2011)] |