| Structure | Name/CAS No. | Articles |
|---|---|---|
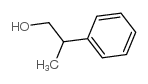 |
2-Phenyl-1-propanol
CAS:1123-85-9 |
|
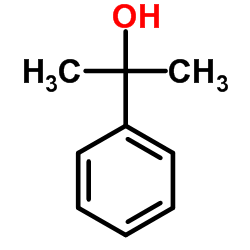 |
α-Cumyl alcohol
CAS:617-94-7 |
|
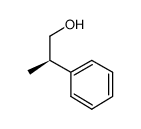 |
(R)-2-phenyl propyl alcohol
CAS:19141-40-3 |
|
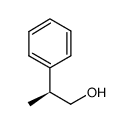 |
(S)-(-)-2-PHENYL-1-PROPANOL
CAS:37778-99-7 |