Process of thermal denaturation of xylanase (XynB) from Clostridium stercorarium F-9.
M Fukumura, A Tanaka, K Sakka, K Ohmiya
Index: Biosci. Biotechnol. Biochem. 59(1) , 47-50, (1995)
Full Text: HTML
Abstract
The thermal denaturation process of Clostridium stercorarium F-9 xylanase (XynB) was studied by monitoring remaining activity and recovered activity of the enzyme. At pH 5.5, aggregation occurred rapidly after the thermal denaturation initiated. The aggregated protein could be dissolved in 8 M urea solution, and the enzyme activity was recovered by diluting the urea. The extent of the recovered activity was gradually decreased with two phases as the reaction time of the thermal denaturation became longer. These results suggested the thermal denaturation process to be as follows: [formula: see text] where N is the native state of the enzyme; D1 is the denatured state of the enzyme that is formed rapidly after the reaction started and can be renatured by the urea treatment, and D2 and D3 are the denatured states of the enzyme that cannot be renatured even by the urea treatment. The rate constants were k1 > 9.2, k2 = 0.33, and k2 = 0.57, and k3 = 0.13 (in min-1 unit).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
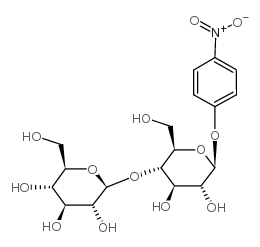 |
4-Nitrophenyl β-D-Cellobioside
CAS:3482-57-3 |
C18H25NO13 |
|
Mechanistic studies of active site mutants of Thermomonospor...
1999-07-27 [Biochemistry 38(30) , 9746-51, (1999)] |
|
Structural changes of cellobiohydrolase I (1,4-beta-D-glucan...
2008-05-01 [Sci. China,. C, Life Sci. 51(5) , 459-69, (2008)] |
|
The competitive inhibition of Trichoderma reesei C30 cellobi...
1990-09-17 [FEBS Lett. 270(1-2) , 143-6, (1990)] |
|
A monovalent anion affected multi-functional cellulase EGX f...
2003-09-01 [Protein Expr. Purif. 31(1) , 108-114, (2003)] |
|
The kinetics ofp-nitrophenyl-β-d-cellobioside hydrolysis and...
2010-01-01 [Carbohydr. Res. 345(17) , 2507-15, (2010)] |