Formation of TEMPOL-hydroxylamine during reaction between TEMPOL and hydroxyl radical: HPLC/ECD study.
Wataru Kudo, Mayumi Yamato, Ken-Ichi Yamada, Yuichi Kinoshita, Takeshi Shiba, Toshiaki Watanabe, Hideo Utsumi
Index: Free Radic. Res. 42(5) , 505-12, (2008)
Full Text: HTML
Abstract
Nitroxyl radicals are important antioxidants that have been used to protect animal tissues from oxidative damage. Their reaction with hydroxyl radical ((*)OH) is generally accepted to be the mechanism of antioxidant function. However, the direct interaction of nitroxyl radicals with (*)OH does not always provide a satisfactory explanation in various pH, because the concentration of hydrogen ion may affect the generation of secondary (*)OH-derived radicals. In the present study, it was confirmed that the reaction between 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) and (*)OH generated TEMPOL-hydroxylamine, 4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPON) and TEMPON-hydroxylamine using HPLC coupled with electrochemical detection. In the absence of NADH, TEMPOL-H may be generated by the reaction with secondary (*)OH-derived radicals in acidic condition. In the presence of NADH, a large proportion of the non-paramagnetic products was TEMPOL-H. Finally, it was clarified that TEMPOL-H was generated during dopamine metabolism, which is believed to be one of the (*)OH sources in pathological processes such as Parkinson's disease.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
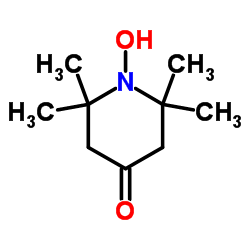 |
4-Oxo-2,2,6,6-tetramethylpiperidinooxy
CAS:2896-70-0 |
C9H16NO2 |
|
Electron paramagnetic resonance line shifts and line shape c...
2012-03-22 [J. Phys. Chem. A 116(11) , 2855-66, (2012)] |
|
A novel analytical method to evaluate directly catalase acti...
2010-09-01 [Free Radic. Res. 44(9) , 1036-43, (2010)] |
|
Neurotoxicity of reactive aldehydes: the concept of "aldehyd...
2006-06-20 [Brain Res. 1095(1) , 190-9, (2006)] |
|
Nitroxide spin exchange due to re-encounter collisions in a ...
2008-08-14 [J. Chem. Phys. 129(6) , 064501, (2008)] |
|
EPR line shifts and line shape changes due to spin exchange ...
2009-04-30 [J. Phys. Chem. A 113(17) , 4930-40, (2009)] |