Monomeric sarcosine oxidase: 2. Kinetic studies with sarcosine, alternate substrates, and a substrate analogue.
Clare V Logan, György Szabadkai, Jenny A Sharpe, David A Parry, Silvia Torelli, Anne-Marie Childs, Marjolein Kriek, Rahul Phadke, Colin A Johnson, Nicola Y Roberts, David T Bonthron, Karen A Pysden, Tamieka Whyte, Iulia Munteanu, A Reghan Foley, Gabrielle Wheway, Katarzyna Szymanska, Subaashini Natarajan, Zakia A Abdelhamed, Joanne E Morgan, Helen Roper, Gijs W E Santen, Erik H Niks, W Ludo van der Pol, Dick Lindhout, Anna Raffaello, Diego De Stefani, Johan T den Dunnen, Yu Sun, Ieke Ginjaar, Caroline A Sewry, Matthew Hurles, Rosario Rizzuto, Michael R Duchen, Francesco Muntoni, Eamonn Sheridan
Index: Biochemistry 39 , 8825-9, (2000)
Full Text: HTML
Abstract
Monomeric sarcosine oxidase (MSOX) is a flavoenzyme that catalyzes the oxidative demethylation of sarcosine (N-methylglycine) to yield glycine, formaldehyde, and hydrogen peroxide. MSOX can oxidize other secondary amino acids (N-methyl-L-alanine, N-ethylglycine, and L-proline), but N,N-dimethylglycine, a tertiary amine, is not a substrate. N-Methyl-L-alanine is a good alternate substrate, exhibiting a k(cat) value (8700 min(-)(1)) similar to sarcosine (7030 min(-)(1)). Turnover with L-proline (k(cat) = 25 min(-)(1)) at 25 degrees C occurs at less than 1% of the rate observed with sarcosine. MSOX is converted to a two-electron reduced form upon anaerobic reduction with sarcosine or L-proline. No evidence for a spectrally detectable intermediate was obtained in reductive half-reaction studies with L-proline. The reductive half-reaction with L-proline at 4 degrees C exhibited saturation kinetics (k(lim) = 6.0 min(-)(1), K(d) = 260 mM) and other features consistent with a mechanism in which a practically irreversible reduction step (E(ox). S --> E(red).P) with a rate constant, k(lim), is preceded by a rapidly attained equilibrium (K(d)) between free E and the E.S complex. Steady-state kinetic studies with sarcosine and N-methyl-L-alanine in the absence or presence of a dead-end inhibitor (pyrrole-2-carboxylate) indicate that catalysis proceeds via a "modified" ping pong mechanism in which oxygen reacts with E(red).P prior to the dissociation of the imino acid product. In this mechanism, double reciprocal plots will appear nearly parallel (as observed) if the reduction step is nearly irreversible. A polar mechanism, involving formation of a covalent 4a-flavin-substrate adduct is one of several plausible mechanisms for sarcosine oxidation. Thiols are known to form similar 4a-flavin adducts. MSOX does not form a 4a-adduct with thioglycolate but does form a charge-transfer complex that undergoes an unanticipated one-electron-transfer reaction to yield the anionic flavin radical.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
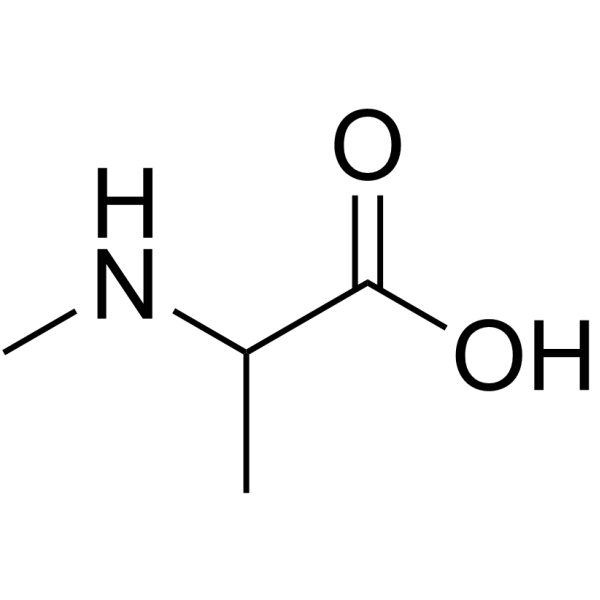 |
N-Me-DL-Ala-OH.HCl
CAS:600-21-5 |
C4H9NO2 | |
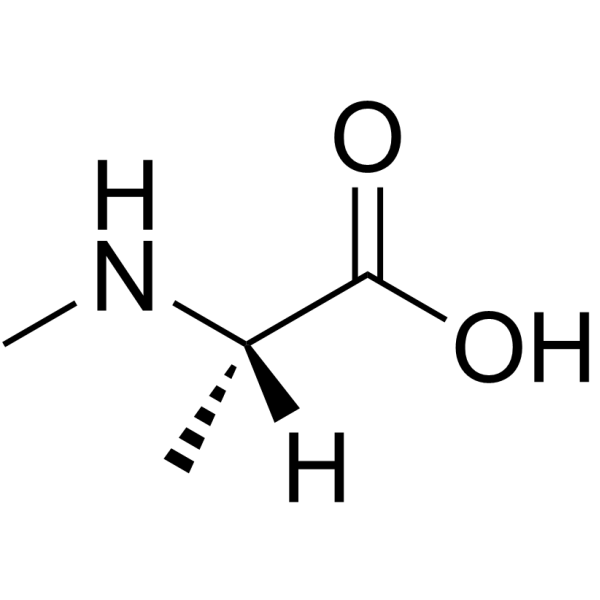 |
H-N-Me-Ala-OH.HCl
CAS:3913-67-5 |
C4H10ClNO2 |
|
1H-NMR and 13C-NMR investigation of complexes of Mn2+ with o...
1996-08-15 [Eur. J. Biochem. 240(1) , 118-24, (1996)] |
|
Transport of L-alanine in cultured human fibroblasts: eviden...
1981-10-01 [J. Cell Physiol. 109(1) , 37-43, (1981)] |
|
Design, synthesis and pharmacological characterization of en...
2010-02-01 [Basic Clin Pharmacol Toxicol. 106 , 106-113, (2010)] |
|
Amino acid efflux in the isolated perfused rat pancreas: tra...
1989-09-01 [J. Physiol. 416 , 485-502, (1989)] |
|
In vitro and in vivo antitumor effects of novel actinomycin ...
2010-04-01 [Peptides 31 , 568-573, (2010)] |