| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
p-Cresol
CAS:106-44-5 |
|
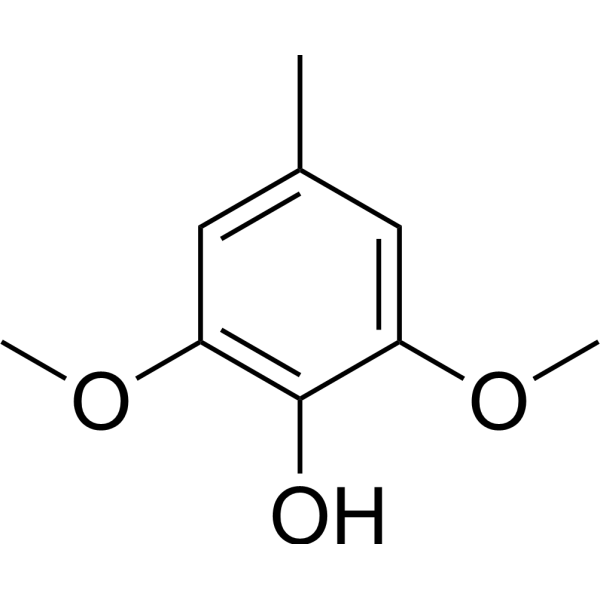 |
Methylsyringol
CAS:6638-05-7 |
|
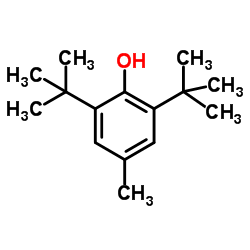 |
Butylated hydroxytoluene
CAS:128-37-0 |