Exclusive production of chloroaniline from chloronitrobenzene over Au/TiO2 and Au/Al2O3.
Fernando Cárdenas-Lizana, Santiago Gómez-Quero, Mark A Keane
Index: ChemSusChem 1(3) , 215-21, (2008)
Full Text: HTML
Abstract
The gas-phase continuous hydrogenation of p-chloronitrobenzene (p-CNB) over 1 mol% Au/TiO2 and Au/Al2O3 was compared for the first time. Both catalysts exhibit 100% selectivity in terms of -NO2 group reduction, resulting in the sole formation of p-chloroaniline (p-CAN). Au/TiO2 exhibited a narrower particle size (1-10 nm) distribution than Au/Al2O3 (1-20 nm) and a smaller surface-area-weighted mean Au size (6 nm versus 9 nm). Au/TiO2 delivered a higher specific hydrogenation rate (by a factor of up to four), a response that is discussed in terms of Au particle size and a possible contribution of the support to p-CNB activation. A CNB isomer reactivity sequence was established, that is, o> p> m, which is attributed to resonance stabilisation effects. The results presented establish a basis for the development of a sustainable alternative route for the production of haloamines.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
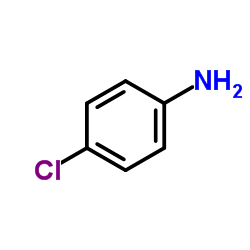 |
4-Chloroaniline
CAS:106-47-8 |
C6H6ClN |
|
cIEF for rapid pKa determination of small molecules: a proof...
2014-10-15 [Eur. J. Pharm. Sci. 63 , 14-21, (2014)] |
|
Calculating virtual log P in the alkane/water system (log P(...
2005-05-05 [J. Med. Chem. 48 , 3269-79, (2005)] |
|
Synthesis and application of hybrid polymer composites based...
2014-01-01 [Molecules 19(5) , 6246-62, (2014)] |
|
QSAR study on permeability of hydrophobic compounds with art...
2007-06-01 [Bioorg. Med. Chem. 15 , 3756-67, (2007)] |
|
Human biomonitoring after chemical incidents and during shor...
2014-12-15 [Toxicol. Lett. 231(3) , 328-36, (2014)] |