Isolation and characterization of a new advanced glycation endproduct of dehydroascorbic acid and lysine.
Ognyan K Argirov, Bin Lin, Paul Olesen, Beryl J Ortwerth
Index: Biochim. Biophys. Acta 1620(1-3) , 235-44, (2003)
Full Text: HTML
Abstract
Proteins are subject of posttranslational modification by sugars and their degradation products in vivo. The process is often referred as glycation. L-Dehydroascorbic acid (DHA), an oxidation product of L-ascorbic acid (vitamin C), is known as a potent glycation agent. A new product of modification of lysine epsilon -amino group by DHA was discovered as a result of the interaction between Boc-Lys and dehydroascorbic acid. The chromatographic and spectral analyses revealed that the structure of the product was 1-(5-ammonio-5-carboxypentyl)-3-oxido-4-(hydroxymethyl)pyridinium. The same compound was isolated from DHA modified calf lens protein after hydrolysis and chromatographic separation. The study confirmed that L-erythrulose is an important intermediate of modification of proteins by DHA. The structure of the reported product and in vitro experiments suggested that L-erythrulose could further transform to L-threose, L-erythrose and glycolaldehyde under conditions similar to physiological. The present study revealed that the modification of epsilon -amino groups of lysine residues by DHA is a complex process and could involve a number of reactive carbonyl species.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
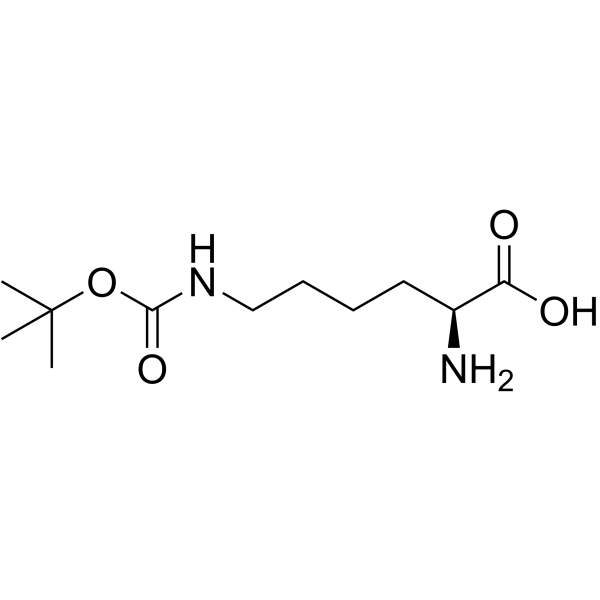 |
Ne-Boc-L-lysine
CAS:2418-95-3 |
C11H22N2O4 |
|
Adding l-lysine derivatives to the genetic code of mammalian...
2008-07-11 [Biochem. Biophys. Res. Commun. 371(4) , 818-22, (2008)] |
|
Evidence that malondialdehyde-derived aminoenimine is not a ...
2001-05-01 [Chem. Res. Toxicol. 14(5) , 473-5, (2001)] |
|
Nanoassembly of surfactants with interfacial drug-interactiv...
2013-01-07 [Mol. Pharm. 10(1) , 187-98, (2013)] |
|
Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in ...
1999-11-15 [Biochem. J. 344 Pt 1 , 109-16, (1999)] |
|
Chain-length dependence for secondary structure formation of...
1984-01-01 [Int. J. Pept. Protein Res. 23(1) , 47-54, (1984)] |