Synthesis of fatty acyl derivatives of 24-epibrassinolide.
Vladimir A Khripach, Vladimir N Zhabinskii, Dmitrii V Tsavlovskii
Index: J. Steroid Biochem. Mol. Biol. 137 , 345-54, (2013)
Full Text: HTML
Abstract
A number of fatty acid (palmitic, myristic and lauric) esters (both 3α- and 3β-isomers) of epibrassinolide has been prepared as reference compounds for metabolic studies. Selective protection of the three of four hydroxyl groups of epibrassinolide was successively performed first as cyclic 22,23-methylboronates and then as 2α-benzyl ethers. α,β-Inversion of C-3 hydroxyl group was achieved through a consecutive oxidation-reduction reactions or by a nucleophilic substitution of the 3α-mesylates. Treatment of the 3α- and 3β-alcohols with palmitic, myristinic or lauric acid chlorides gave the corresponding esters. The hydrolysis of 22,23-methylboronates was performed after their transformation into 2-hydroxy-1,3,2-dioxaborolanes using a cation exchange column with DOWEX 50WX8 in NH4(+) form. Hydrogenolysis of the benzyl ethers catalyzed by palladium yielded the target compounds. This article is part of a Special Issue entitled "Synthesis and biological testing of steroid derivatives as inhibitors". Copyright © 2013 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
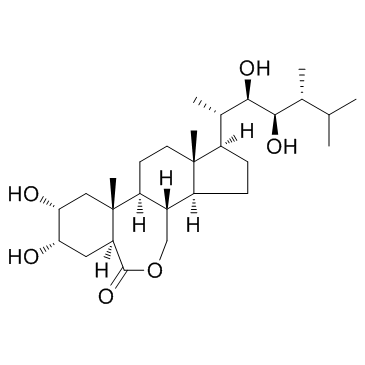 |
Epibrassinolide
CAS:78821-43-9 |
C28H48O6 |
|
Organ-specific effects of brassinosteroids on stomatal produ...
2015-03-01 [J. Integr. Plant Biol. 57(3) , 247-55, (2015)] |
|
Conifer somatic embryogenesis: improvements by supplementati...
2015-02-01 [Tree Physiol. 35 , 209-24, (2015)] |
|
Proteomic Analysis of Microtubule Interacting Proteins over ...
2015-10-01 [Plant Cell 27 , 2709-26, (2015)] |
|
The poplar basic helix-loop-helix transcription factor BEE3 ...
2015-06-19 [Biochem. Biophys. Res. Commun. 462 , 64-70, (2015)] |
|
Epibrassinolide-induced apoptosis regardless of p53 expressi...
2014-12-01 [Prostate 74(16) , 1622-33, (2014)] |