Structural insight into potential cold adaptation mechanism through a psychrophilic glycoside hydrolase family 10 endo-β-1,4-xylanase.
Yingying Zheng, Yujie Li, Weidong Liu, Chun-Chi Chen, Tzu-Ping Ko, Miao He, Zhongxia Xu, Meixia Liu, Huiying Luo, Rey-Ting Guo, Bin Yao, Yanhe Ma
Index: J. Struct. Biol. 193 , 206-11, (2016)
Full Text: HTML
Abstract
The cold-adapted xylanases can catalyze at low temperature and hold great potential in food industry applications. Here we describe the first crystal structure of a cold-adapted glycoside hydrolase (GH) family 10 xylanase XynGR40 and its complex with xylobiose at 2.15 and 2.50Å resolution. The enzyme folds into a typical GH10 (β/α)8 TIM-barrel, with E132 and E243 serving as the catalytic residues. The xylobiose was observed to occupy the -1 and -2 subsites. Structural comparison with a thermophilic GH10 xylanase highlighting various parameters that may explain the cold adaptation features were analyzed. Synergistic effects of the increased exposure of hydrophobic residues, the higher flexibility of substrate-binding residues, more flexible loops, and the ratios of special amino acid residues, may result in the cold adaptation of XynGR40. Copyright © 2015 Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
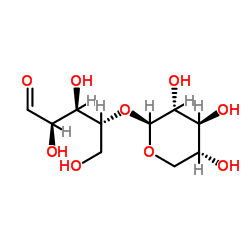 |
Xylobiose
CAS:6860-47-5 |
C10H18O9 |
|
Characterization of the biochemical properties of recombinan...
2016-04-01 [Bioprocess Biosyst. Eng. 39 , 677-84, (2016)] |
|
Characterization of (Glucurono)arabinoxylans from Oats Using...
2015-12-23 [J. Agric. Food Chem. 63 , 10822-30, (2015)] |
|
Comparative Analysis of End Point Enzymatic Digests of Arabi...
2012-01-01 [Metabolites 2 , 959-82, (2014)] |
|
A novel highly thermostable xylanase stimulated by Ca2+ from...
2013-01-01 [Biotechnol. Biofuels 6 , 26, (2013)] |
|
Purification and biochemical characterization of a specific ...
2009-01-01 [J. Insect Sci. 9 , 4, (2009)] |