| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
1,3-bis(2-cyclohexylnaphthalen-1-yl)imidazolinium tetrafluoroborate
CAS:1208220-06-7 |
|
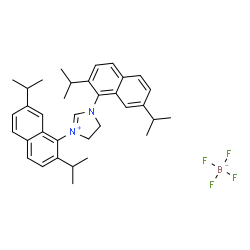 |
1,3-Bis(2,7-diisopropylnaphthalen-1-yl)imidazolinium tetrafluoroborate
CAS:1025030-97-0 |