| Structure | Name/CAS No. | Articles |
|---|---|---|
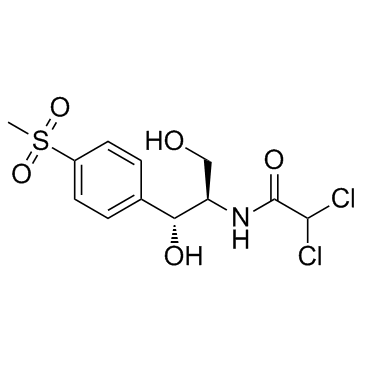 |
Thiamphenicol
CAS:15318-45-3 |
|
 |
L-cysteine
CAS:52-90-4 |
|
 |
Endoproteinase Arg-C
CAS:82047-85-6 |