The cobalt way to angucyclinones: asymmetric total synthesis of the antibiotics (+)-rubiginone B2, (-)-tetrangomycin, and (-)-8-O-methyltetrangomycin.
Christian Kesenheimer, Aris Kalogerakis, Anja Meissner, Ulrich Groth
Index: Chemistry 16(29) , 8805-21, (2010)
Full Text: HTML
Abstract
A cobalt(I)-mediated convergent and asymmetric total synthesis of angucyclinones with an aromatic B ring has been developed. In the course of our research, we synthesized three naturally occurring anguclinone derivatives, namely, (+)-rubiginone B(2) (1), (-)-8-O-methyltetrangomycin (2), and (-)-tetrangomycin (3). By combining 3-hydroxybenzoic acid, 3-methoxybenzoic acid, citronellal, and geraniol as starting materials in a convergent way, we were able to synthesize chiral triyne chains, which were cyclized with [CpCo(C(2)H(4))(2)] (Cp=cyclopentadienyl) by means of an intramolecular [2+2+2] cycloaddition to their corresponding tetrahydrobenzo[a]anthracenes. Successive oxidation and deprotection steps led to the above-mentioned natural products 1-3.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
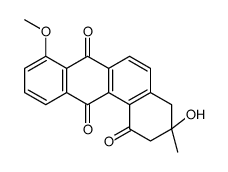 |
MM 47755
CAS:117620-87-8 |
C20H16O5 |
|
Angucyclinone antibiotics: total syntheses of YM-181741, (+)...
2007-08-03 [J. Org. Chem. 72(16) , 6116-26, (2007)] |
|
Total synthesis of (-)-8-O-methyltetrangomycin (MM 47755).
2006-06-08 [Org. Lett. 8(12) , 2507-10, (2006)] |
|
Biological activities of deoxyspergualin in autoimmune disea...
1988-09-01 [J. Antibiot. 41(9) , 1253-9, (1988)] |
|
Search method for inhibitors of Staphyloxanthin production b...
2012-01-01 [Biol. Pharm. Bull. 35(1) , 48-53, (2012)] |
|
MM 47755, a new benz[a]anthracene antibiotic from a streptom...
1989-04-01 [J. Antibiot. 42(4) , 627-8, (1989)] |